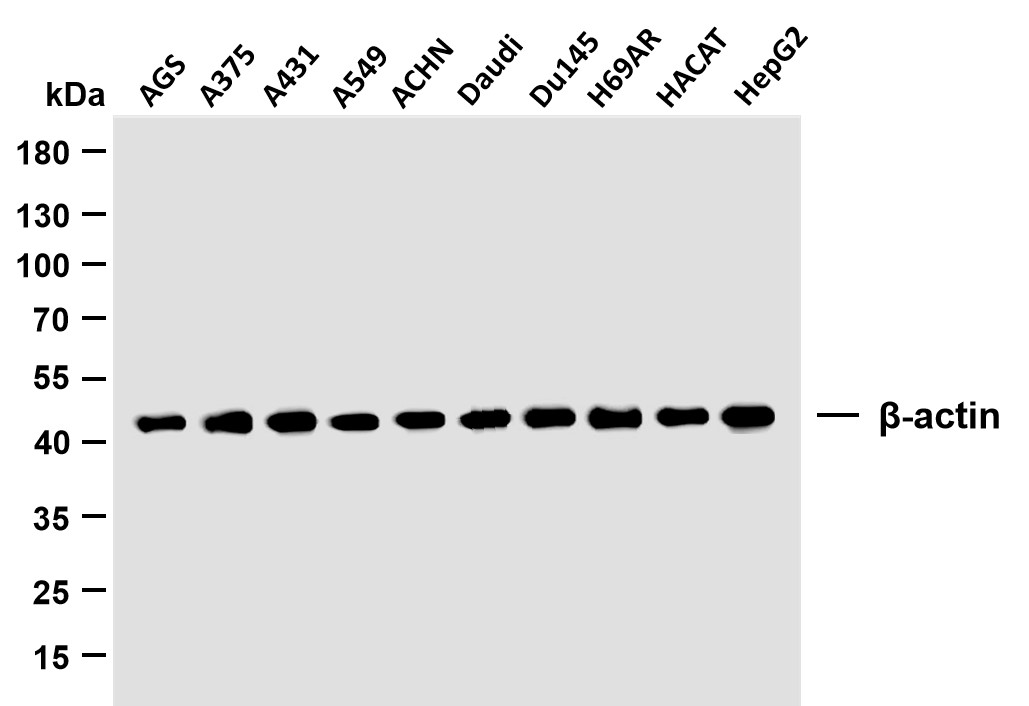
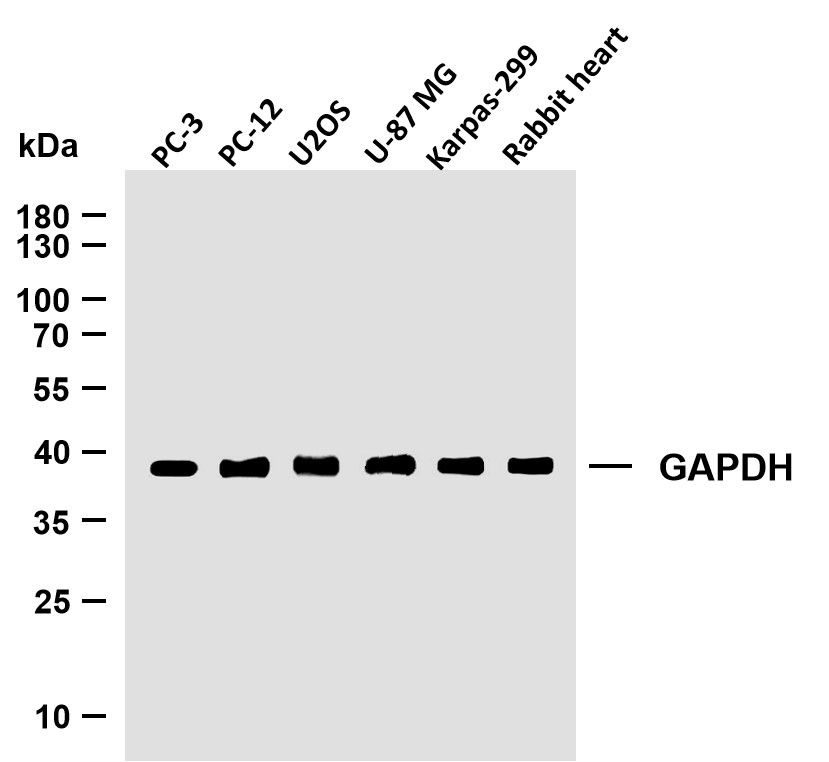
Catalog: YT6443
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$150.00
In stock
0
Add to cart


Collected


Collect
Main Information
Target
DYH2
Host Species
Rabbit
Reactivity
Human, Mouse
Applications
IHC, IF
MW
487kD (Calculated)
Conjugate/Modification
Unmodified
Detailed Information
Recommended Dilution Ratio
IHC 1:50-200; IF 1:50-200
Formulation
Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
Specificity
This antibody detects endogenous levels of DYH2 at Human/Mouse
Purification
The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
Storage
-15°C to -25°C/1 year(Do not lower than -25°C)
Concentration
1 mg/ml
MW(Calculated)
487kD
Modification
Unmodified
Clonality
Polyclonal
Isotype
IgG
Related Products
Antigen&Target Information
Immunogen:
Synthesized peptide derived from human DYH2 AA range: 329-379
show all
Specificity:
This antibody detects endogenous levels of DYH2 at Human/Mouse
show all
Gene Name:
DNAH2 DNAHC2 DNHD3 KIAA1503
show all
Protein Name:
DYH2
show all
Background:
Dyneins are microtubule-associated motor protein complexes composed of several heavy, light, and intermediate chains. The axonemal dyneins, found in cilia and flagella, are components of the outer and inner dynein arms attached to the peripheral microtubule doublets. DNAH2 is an axonemal inner arm dynein heavy chain (Chapelin et al., 1997 [PubMed 9256245]).[supplied by OMIM, Mar 2008],
show all
Function:
Domain:Dynein heavy chains probably consist of an N-terminal stem (which binds cargo and interacts with other dynein components), and the head or motor domain. The motor contains six tandemly-linked AAA domains in the head, which form a ring. A stalk-like structure (formed by two of the coiled coil domains) protrudes between AAA 4 and AAA 5 and terminates in a microtubule-binding site. A seventh domain may also contribute to this ring; it is not clear whether the N-terminus or the C-terminus forms this extra domain. There are four well-conserved and two non-conserved ATPase sites, one per AAA domain. Probably only one of these (within AAA 1) actually hydrolyzes ATP, the others may serve a regulatory function.,Function:Force generating protein of respiratory cilia. Produces force towards the minus ends of microtubules. Dynein has ATPase activity; the force-producing power stroke is thought to occur on release of ADP. Involved in sperm motility; implicated in sperm flagellar assembly.,similarity:Belongs to the dynein heavy chain family.,similarity:Contains 5 LRR (leucine-rich) repeats.,similarity:Contains 5 TPR repeats.,subunit:Consists of at least two heavy chains and a number of intermediate and light chains.,tissue specificity:Expressed primarily in trachea and testis, 2 tissues containing axonemal structures. Also expressed in lung.,
show all
Cellular Localization:
Cytoplasm, cytoskeleton, cilium axoneme . Cytoplasm, cytoskeleton, flagellum axoneme .
show all
Tissue Expression:
Expressed primarily in trachea and testis, 2 tissues containing axonemal structures. Also expressed in lung. Expressed in spermatozoa (at protein level) (PubMed:31178125).
show all
Research Areas:
>>Amyotrophic lateral sclerosis ;
>>Huntington disease ;
>>Pathways of neurodegeneration - multiple diseases
>>Huntington disease ;
>>Pathways of neurodegeneration - multiple diseases
show all
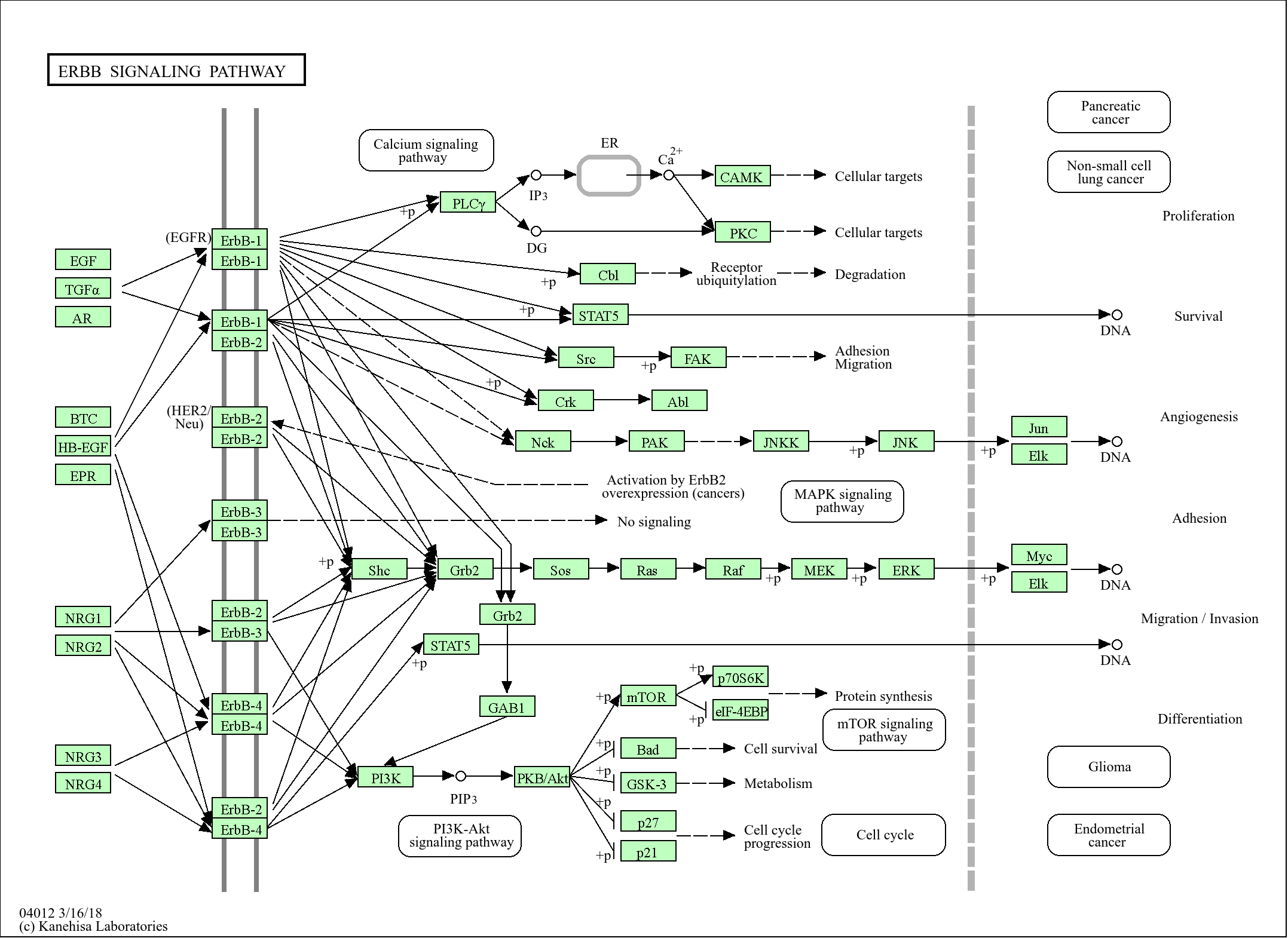
Signaling Pathway
Reference Citation({{totalcount}})
Catalog: YT6443
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$150.00
In stock
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}