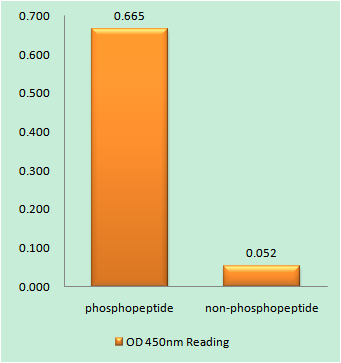
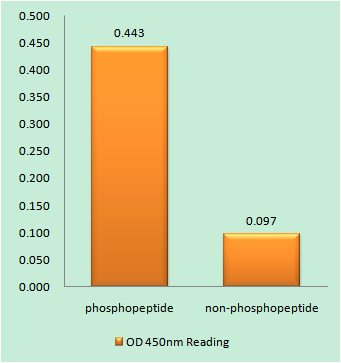
Catalog: YP0228
Size
Price
Status
Qty.
200μL
$600.00
In stock
0
100μL
$340.00
In stock
0
50μL
$190.00
In stock
0
Add to cart


Collected


Collect
Main Information
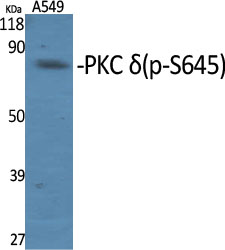
Target
PKC δ
Host Species
Rabbit
Reactivity
Human, Mouse, Rat
Applications
WB, IHC, IF, ELISA
MW
78kD (Observed)
Conjugate/Modification
Phospho
Detailed Information
Recommended Dilution Ratio
WB 1:500-1:2000; IHC 1:100-1:300; ELISA 1:20000; IF 1:50-200
Formulation
Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
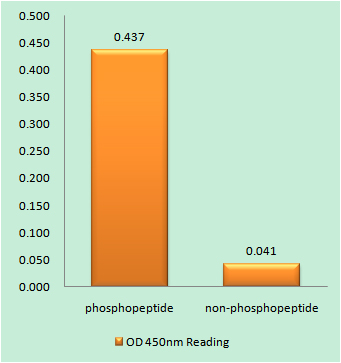
Specificity
Phospho-PKC δ (T507) Polyclonal Antibody detects endogenous levels of PKC δ protein only when phosphorylated at T507.The name of modified sites may be influenced by many factors, such as species (the modified site was not originally found in human samples) and the change of protein sequence (the previous protein sequence is incomplete, and the protein sequence may be prolonged with the development of protein sequencing technology). When naming, we will use the "numbers" in historical reference to keep the sites consistent with the reports. The antibody binds to the following modification sequence (lowercase letters are modification sites):AStFC
Purification
The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
Storage
-15°C to -25°C/1 year(Do not lower than -25°C)
Concentration
1 mg/ml
MW(Observed)
78kD
Modification
Phospho
Clonality
Polyclonal
Isotype
IgG
Related Products
Antigen&Target Information
Immunogen:
The antiserum was produced against synthesized peptide derived from human PKC delta around the phosphorylation site of Thr505. AA range:471-520
show all
Specificity:
Phospho-PKC δ (T507) Polyclonal Antibody detects endogenous levels of PKC δ protein only when phosphorylated at T507.The name of modified sites may be influenced by many factors, such as species (the modified site was not originally found in human samples) and the change of protein sequence (the previous protein sequence is incomplete, and the protein sequence may be prolonged with the development of protein sequencing technology). When naming, we will use the "numbers" in historical reference to keep the sites consistent with the reports. The antibody binds to the following modification sequence (lowercase letters are modification sites):AStFC
show all
Gene Name:
PRKCD
show all
Protein Name:
Protein kinase C delta type
show all
Other Name:
PRKCD ;
Protein kinase C delta type ;
Tyrosine-protein kinase PRKCD ;
nPKC-delta
Protein kinase C delta type ;
Tyrosine-protein kinase PRKCD ;
nPKC-delta
show all
Database Link:
Background:
Protein kinase C (PKC) is a family of serine- and threonine-specific protein kinases that can be activated by calcium and the second messenger diacylglycerol. PKC family members phosphorylate a wide variety of protein targets and are known to be involved in diverse cellular signaling pathways. PKC family members also serve as major receptors for phorbol esters, a class of tumor promoters. Each member of the PKC family has a specific expression profile and is believed to play distinct roles in cells. The protein encoded by this gene is one of the PKC family members. Studies both in human and mice demonstrate that this kinase is involved in B cell signaling and in the regulation of growth, apoptosis, and differentiation of a variety of cell types. Alternatively spliced transcript variants encoding the same protein have been observed. [provided by RefSeq, Jul 2008],
show all
Function:
Catalytic activity:ATP + a protein = ADP + a phosphoprotein.,Domain:The C1 domain, containing the phorbol ester/DAG-type region 1 (C1A) and 2 (C1B), is the diacylglycerol sensor.,Domain:The C2 domain is a non-calcium binding domain. It binds proteins containing phosphotyrosine in a sequence-specific manner.,enzyme regulation:Three specific sites; Thr-507 (activation loop of the kinase domain), Ser-645 (turn motif) and Ser-664 (hydrophobic region), need to be phosphorylated for its full activation.,Function:This is calcium-independent, phospholipid-dependent, serine- and threonine-specific enzyme. PKC is activated by diacylglycerol which in turn phosphorylates a range of cellular proteins. PKC also serves as the receptor for phorbol esters, a class of tumor promoters. May play a role in antigen-dependent control of B-cell function. Phosphorylates MUC1 in the C-terminal and regulates the interaction between MUC1 and beta-catenin.,PTM:Phosphorylated on Thr-507, within the activation loop. Autophosphorylated and/or phosphorylated. Although the Thr-507 phosphorylation occurs it is not a prerequisite for enzymatic activity.,similarity:Belongs to the protein kinase superfamily. AGC Ser/Thr protein kinase family. PKC subfamily.,similarity:Contains 1 AGC-kinase C-terminal domain.,similarity:Contains 1 C2 domain.,similarity:Contains 1 protein kinase domain.,similarity:Contains 2 phorbol-ester/DAG-type zinc fingers.,subunit:Interacts with PDK1, RAD9A, CDCP1 and MUC1.,
show all
Cellular Localization:
Cytoplasm . Cytoplasm, perinuclear region . Nucleus . Cell membrane ; Peripheral membrane protein . Mitochondrion . Endomembrane system . Translocates to the mitochondria upon apoptotic stimulation. Upon activation, translocates to the plasma membrane followed by partial location to the endolysosomes (PubMed:17303575). .
show all
Tissue Expression:
Epithelium,Hippocampus,Liver,Platelet,Skin,
show all
Research Areas:
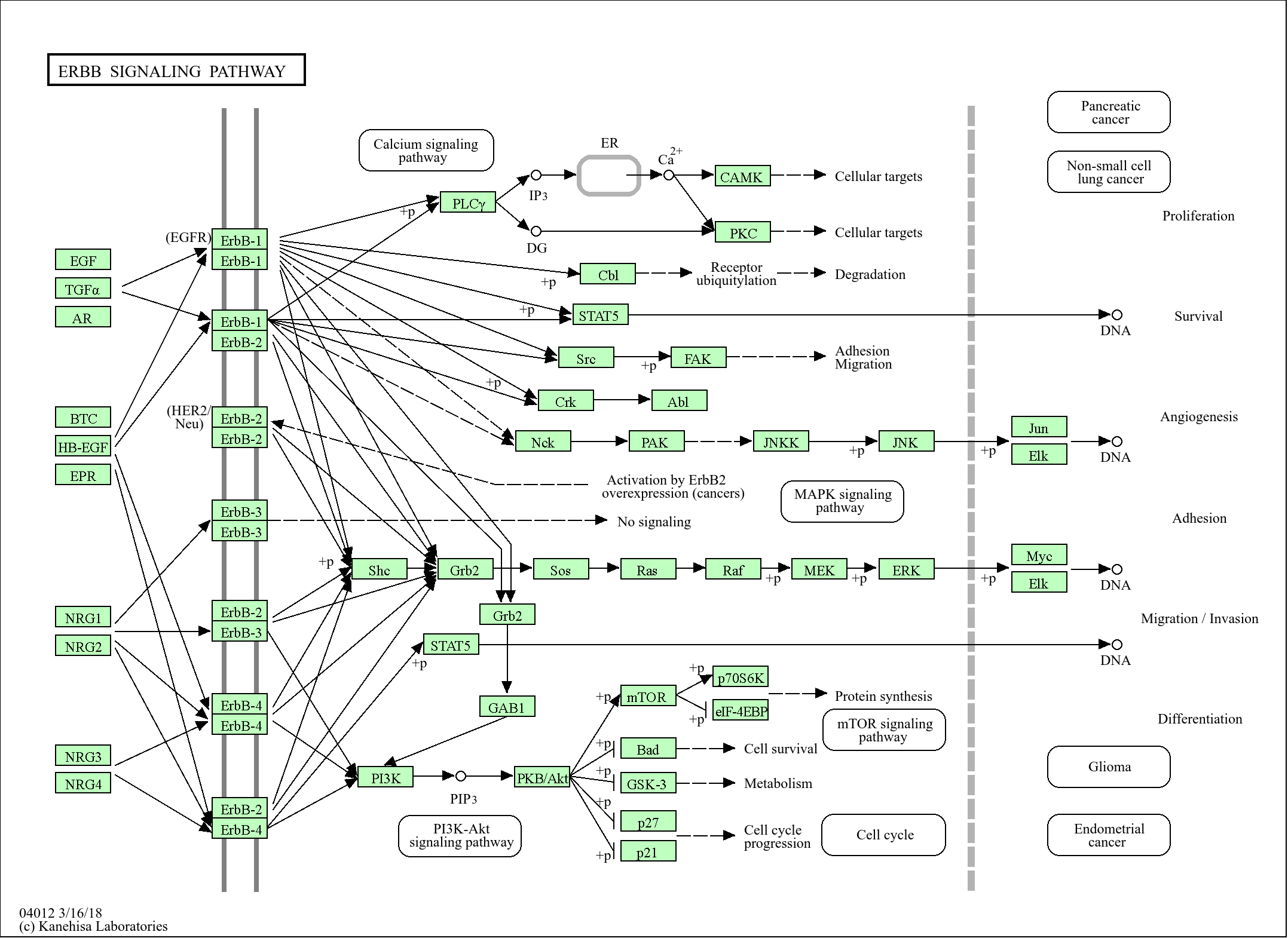
>>Chemokine signaling pathway ;
>>Autophagy - animal ;
>>Vascular smooth muscle contraction ;
>>NOD-like receptor signaling pathway ;
>>C-type lectin receptor signaling pathway ;
>>Fc gamma R-mediated phagocytosis ;
>>Neurotrophin signaling pathway ;
>>Inflammatory mediator regulation of TRP channels ;
>>GnRH signaling pathway ;
>>Estrogen signaling pathway ;
>>Type II diabetes mellitus ;
>>Insulin resistance ;
>>AGE-RAGE signaling pathway in diabetic complications ;
>>Prion disease ;
>>Shigellosis ;
>>Chemical carcinogenesis - reactive oxygen species ;
>>Diabetic cardiomyopathy
>>Autophagy - animal ;
>>Vascular smooth muscle contraction ;
>>NOD-like receptor signaling pathway ;
>>C-type lectin receptor signaling pathway ;
>>Fc gamma R-mediated phagocytosis ;
>>Neurotrophin signaling pathway ;
>>Inflammatory mediator regulation of TRP channels ;
>>GnRH signaling pathway ;
>>Estrogen signaling pathway ;
>>Type II diabetes mellitus ;
>>Insulin resistance ;
>>AGE-RAGE signaling pathway in diabetic complications ;
>>Prion disease ;
>>Shigellosis ;
>>Chemical carcinogenesis - reactive oxygen species ;
>>Diabetic cardiomyopathy
show all
Signaling Pathway
Cellular Processes >> Transport and catabolism >> Autophagy - animal
Organismal Systems >> Immune system >> NOD-like receptor signaling pathway
Organismal Systems >> Immune system >> Fc gamma R-mediated phagocytosis
Organismal Systems >> Immune system >> Chemokine signaling pathway
Organismal Systems >> Endocrine system >> GnRH signaling pathway
Organismal Systems >> Endocrine system >> Estrogen signaling pathway
Organismal Systems >> Circulatory system >> Vascular smooth muscle contraction
Organismal Systems >> Nervous system >> Neurotrophin signaling pathway
Organismal Systems >> Sensory system >> Inflammatory mediator regulation of TRP channels
Human Diseases >> Neurodegenerative disease >> Prion disease
Reference Citation({{totalcount}})
Catalog: YP0228
Size
Price
Status
Qty.
200μL
$600.00
In stock
0
100μL
$340.00
In stock
0
50μL
$190.00
In stock
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}