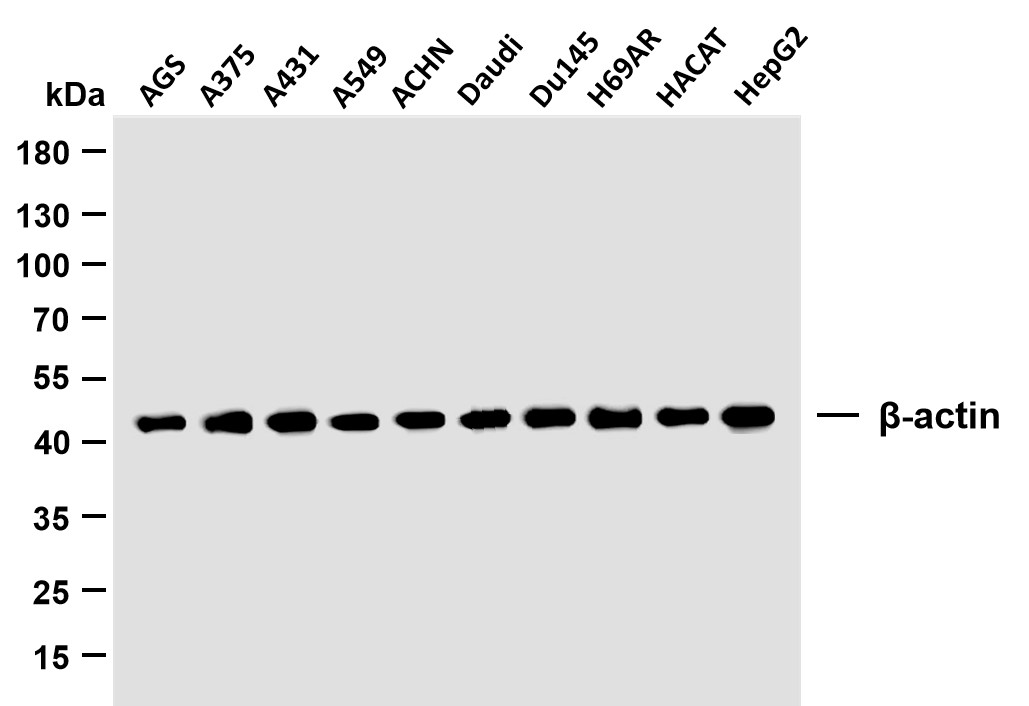
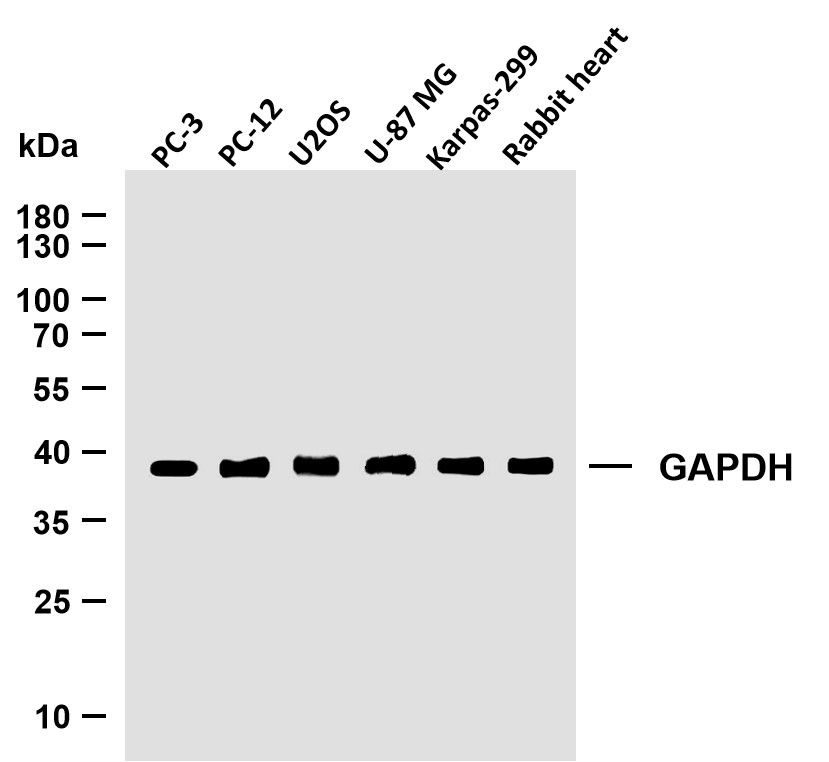
Catalog: YN2440
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$150.00
In stock
0
Add to cart


Collected


Collect
Main Information
Target
NRG4
Host Species
Rabbit
Reactivity
Human, Mouse
Applications
WB, ELISA
MW
12kD (Observed)
Conjugate/Modification
Unmodified
Detailed Information
Recommended Dilution Ratio
WB 1:500-2000; ELISA 1:5000-20000
Formulation
Liquid in PBS containing 50% glycerol, and 0.02% sodium azide.
Specificity
NRG4 Polyclonal Antibody detects endogenous levels of protein.
Purification
The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
Storage
-15°C to -25°C/1 year(Do not lower than -25°C)
Concentration
1 mg/ml
MW(Observed)
12kD
Modification
Unmodified
Clonality
Polyclonal
Isotype
IgG
Related Products
Antigen&Target Information
Immunogen:
Synthesized peptide derived from human protein . at AA range: 11-60
show all
Specificity:
NRG4 Polyclonal Antibody detects endogenous levels of protein.
show all
Gene Name:
NRG4
show all
Protein Name:
Pro-neuregulin-4, membrane-bound isoform (Pro-NRG4) [Cleaved into: Neuregulin-4 (NRG-4)]
show all
Background:
The neuregulins, including NRG4, activate type-1 growth factor receptors (see EGFR; MIM 131550) to initiating cell-to-cell signaling through tyrosine phosphorylation (Harari et al., 1999 [PubMed 10348342]).[supplied by OMIM, Mar 2008],
show all
Function:
Domain:ERBB receptor binding is elicited entirely by the EGF-like domain.,Domain:The cytoplasmic domain may be involved in the regulation of trafficking and proteolytic processing. Regulation of the proteolytic processing involves initial intracellular domain dimerization.,Function:Low affinity ligand for the ERBB4 tyrosine kinase receptor. Concomitantly recruits ERBB1 and ERBB2 coreceptors, resulting in ligand-stimulated tyrosine phosphorylation and activation of the ERBB receptors. Does not bind to the ERBB1, ERBB2 and ERBB3 receptors.,PTM:Extensive glycosylation precedes the proteolytic cleavage.,PTM:Proteolytic cleavage close to the plasma membrane on the external face leads to the release of the soluble growth factor form.,similarity:Belongs to the neuregulin family.,similarity:Contains 1 EGF-like domain.,subcellular location:Does not seem to be active.,
show all
Cellular Localization:
[Pro-neuregulin-4, membrane-bound isoform]: Cell membrane ; Single-pass type I membrane protein . Does not seem to be active. .; [Neuregulin-4]: Secreted .
show all
Tissue Expression:
Breast,Liver,Prostate,
show all
Research Areas:
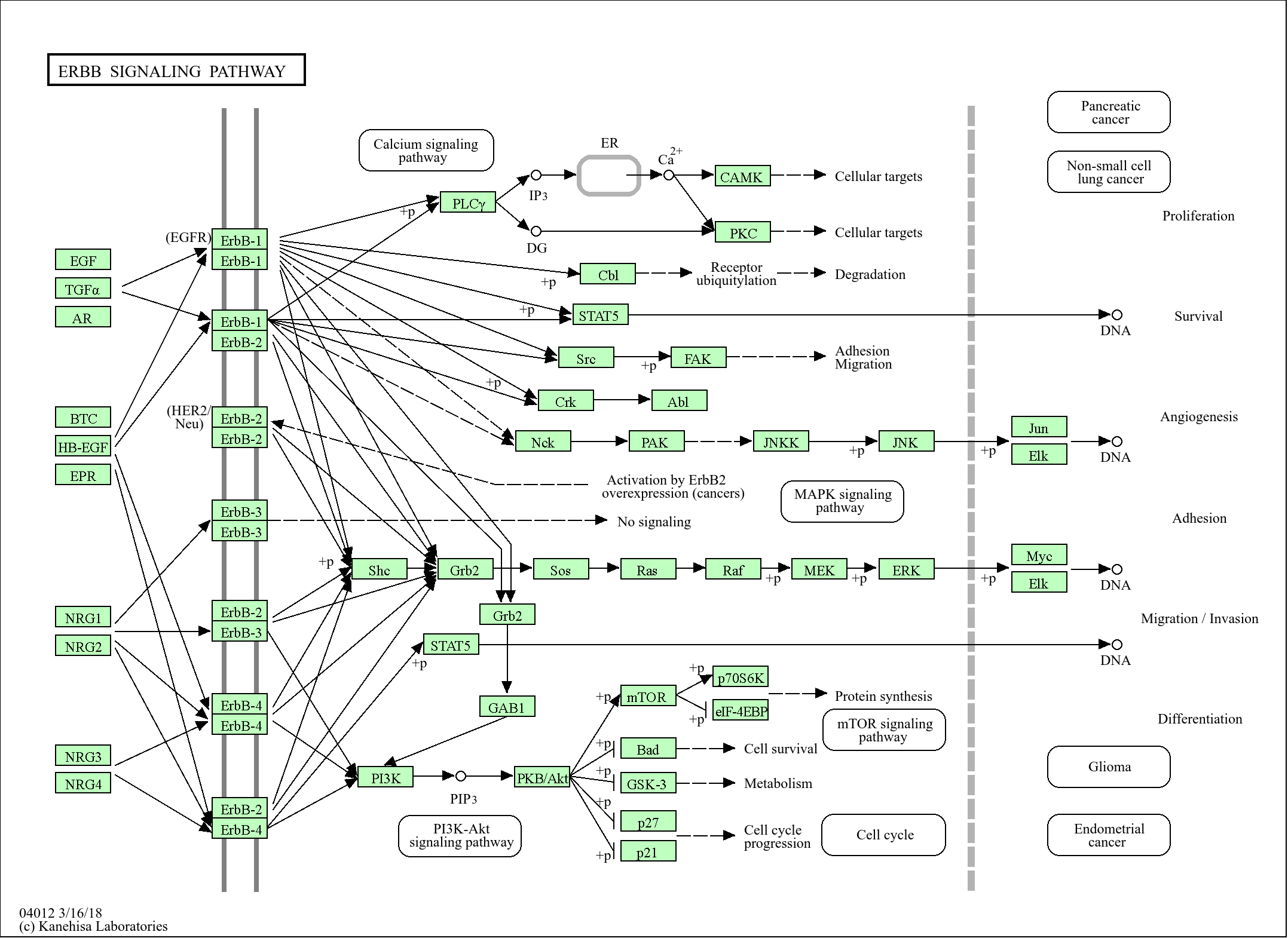
>>ErbB signaling pathway ;
>>Amyotrophic lateral sclerosis
>>Amyotrophic lateral sclerosis
show all
Signaling Pathway
Reference Citation({{totalcount}})
Catalog: YN2440
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$150.00
In stock
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}