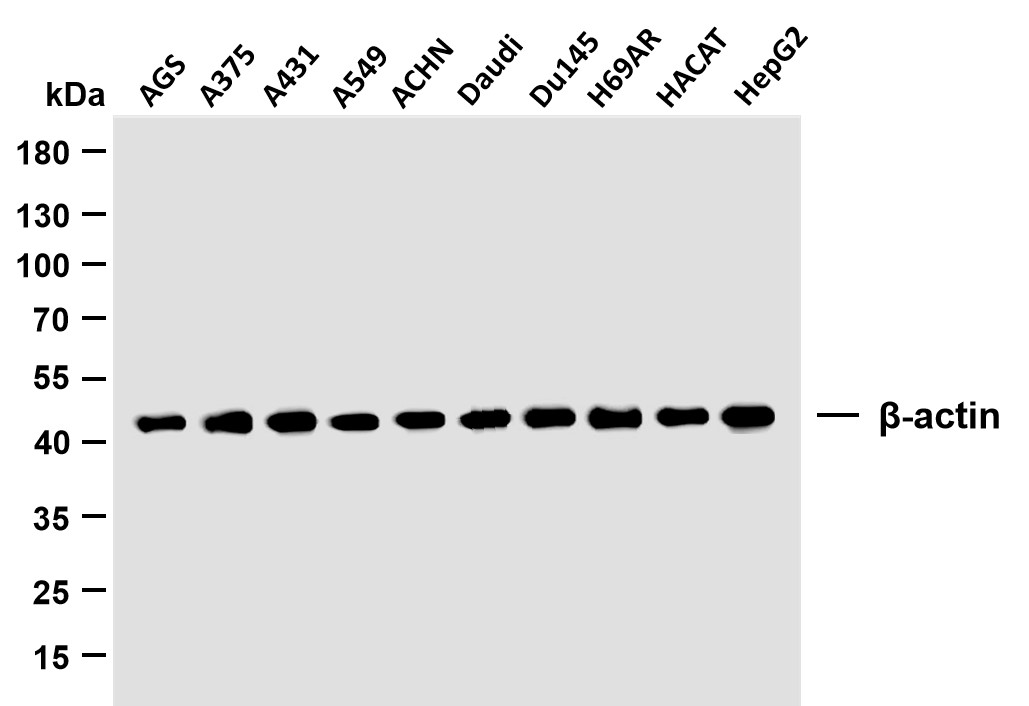
Catalog: YN1858
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$150.00
In stock
0
Add to cart


Collected


Collect
Main Information
Target
ANPRA
Host Species
Rabbit
Reactivity
Human, Mouse, Rat
Applications
WB, ELISA
MW
116kD (Observed)
Conjugate/Modification
Unmodified
Detailed Information
Recommended Dilution Ratio
WB 1:500-2000; ELISA 1:5000-20000
Formulation
Liquid in PBS containing 50% glycerol,0.5% BSA and 0.02% sodium azide.
Specificity
ANPRA Polyclonal Antibody detects endogenous levels of protein.
Purification
The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
Storage
-15°C to -25°C/1 year(Do not lower than -25°C)
Concentration
1 mg/ml
MW(Observed)
116kD
Modification
Unmodified
Clonality
Polyclonal
Isotype
IgG
Related Products
Antigen&Target Information
Immunogen:
Synthesized peptide derived from part region of human protein
show all
Specificity:
ANPRA Polyclonal Antibody detects endogenous levels of protein.
show all
Gene Name:
NPR1 ANPRA
show all
Protein Name:
Atrial natriuretic peptide receptor 1 (Atrial natriuretic peptide receptor type A) (ANP-A) (ANPR-A) (NPR-A) (Guanylate cyclase A) (GC-A)
show all
Background:
Guanylyl cyclases, catalyzing the production of cGMP from GTP, are classified as soluble and membrane forms (Garbers and Lowe, 1994 [PubMed 7982997]). The membrane guanylyl cyclases, often termed guanylyl cyclases A through F, form a family of cell-surface receptors with a similar topographic structure: an extracellular ligand-binding domain, a single membrane-spanning domain, and an intracellular region that contains a protein kinase-like domain and a cyclase catalytic domain. GC-A and GC-B function as receptors for natriuretic peptides; they are also referred to as atrial natriuretic peptide receptor A (NPR1) and type B (NPR2; MIM 108961). Also see NPR3 (MIM 108962), which encodes a protein with only the ligand-binding transmembrane and 37-amino acid cytoplasmic domains. NPR1 is a membrane-bound guanylate cyclase that serves as the receptor for both atrial and brain natriuretic peptides (A
show all
Function:
Catalytic activity:GTP = 3',5'-cyclic GMP + diphosphate.,Function:Receptor for atrial natriuretic peptide. Has guanylate cyclase activity on binding of ANF.,miscellaneous:There seem to be at least three natriuretic peptide hormone receptors: two with guanylate cyclase activity (NPR1/ANP-A and NPR2/ANP-B) and one (NPR3/ANP-C) which is probably responsible for the clearance of natriuretic peptides from the circulation without a role in signal transduction.,PTM:Phosphorylation of the protein kinase-like domain is required for full activation by ANP.,similarity:Belongs to the adenylyl cyclase class-4/guanylyl cyclase family.,similarity:Contains 1 guanylate cyclase domain.,similarity:Contains 1 protein kinase domain.,
show all
Cellular Localization:
Membrane; Single-pass type I membrane protein.
show all
Tissue Expression:
show all
Research Areas:
>>Purine metabolism ;
>>Metabolic pathways ;
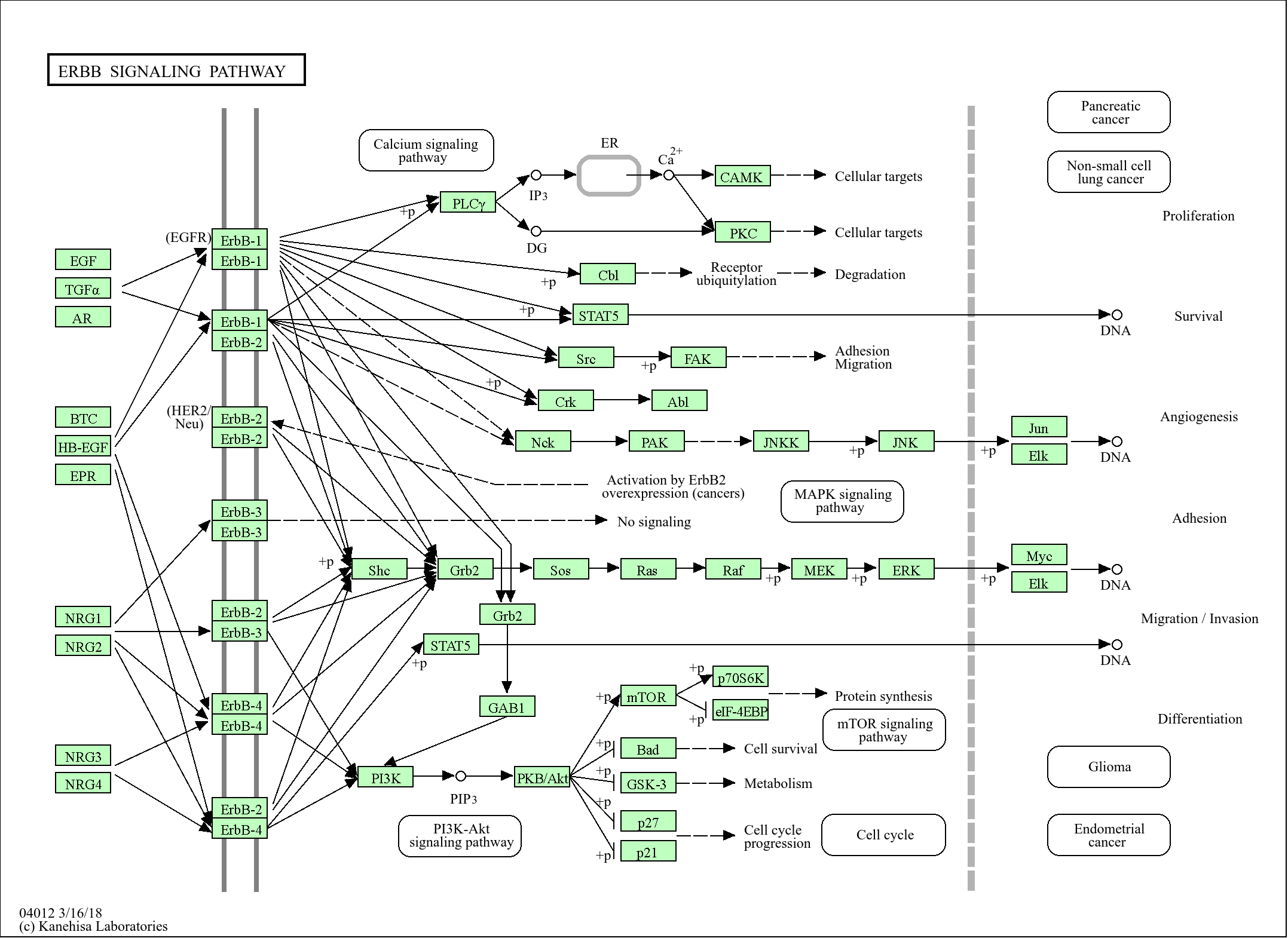
>>cGMP-PKG signaling pathway ;
>>cAMP signaling pathway ;
>>Vascular smooth muscle contraction ;
>>Thermogenesis ;
>>Oxytocin signaling pathway ;
>>Regulation of lipolysis in adipocytes ;
>>Renin secretion ;
>>Aldosterone synthesis and secretion
>>Metabolic pathways ;
>>cGMP-PKG signaling pathway ;
>>cAMP signaling pathway ;
>>Vascular smooth muscle contraction ;
>>Thermogenesis ;
>>Oxytocin signaling pathway ;
>>Regulation of lipolysis in adipocytes ;
>>Renin secretion ;
>>Aldosterone synthesis and secretion
show all
Signaling Pathway
Organismal Systems >> Endocrine system >> Oxytocin signaling pathway
Organismal Systems >> Endocrine system >> Aldosterone synthesis and secretion
Organismal Systems >> Circulatory system >> Vascular smooth muscle contraction
Environmental Information Processing >> Signal transduction >> cAMP signaling pathway
Environmental Information Processing >> Signal transduction >> cGMP-PKG signaling pathway
Reference Citation({{totalcount}})
Catalog: YN1858
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$150.00
In stock
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}