Catalog: YN0086
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$150.00
In stock
0
Add to cart


Collected


Collect
Main Information
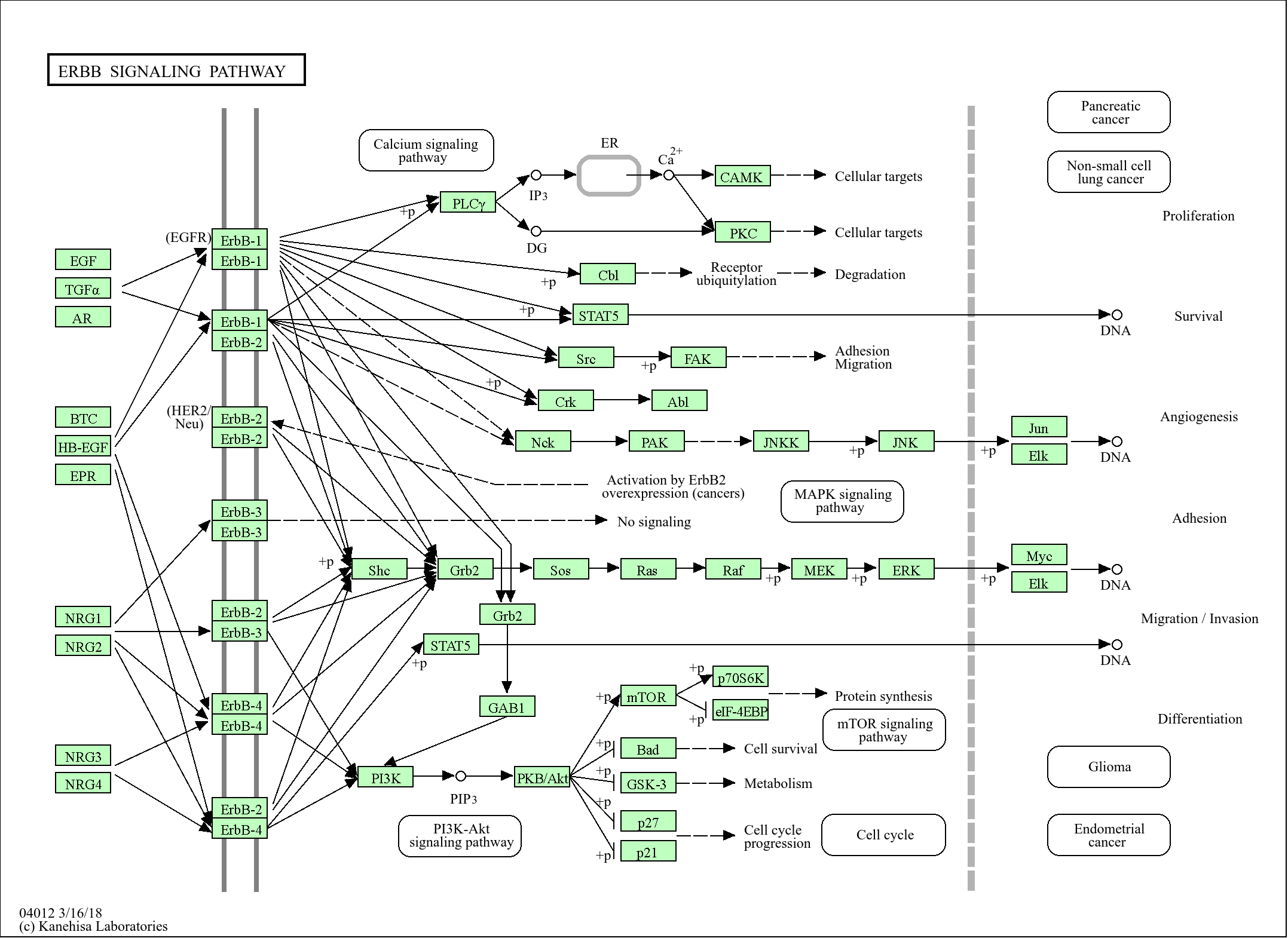
Target
DUS14
Host Species
Rabbit
Reactivity
Human, Mouse
Applications
WB, ELISA
MW
21kD (Observed)
Conjugate/Modification
Unmodified
Detailed Information
Recommended Dilution Ratio
WB 1:500-2000; ELISA 1:5000-20000
Formulation
Liquid in PBS containing 50% glycerol,0.5% BSA and 0.02% sodium azide.
Specificity
DUS14 Polyclonal Antibody detects endogenous levels of protein.
Purification
The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
Storage
-15°C to -25°C/1 year(Do not lower than -25°C)
Concentration
1 mg/ml
MW(Observed)
21kD
Modification
Unmodified
Clonality
Polyclonal
Isotype
IgG
Related Products
Antigen&Target Information
Immunogen:
Synthesized peptide derived from human protein . at AA range: 120-200
show all
Specificity:
DUS14 Polyclonal Antibody detects endogenous levels of protein.
show all
Gene Name:
DUSP14 MKP6
show all
Protein Name:
Dual specificity protein phosphatase 14 (MKP-1-like protein tyrosine phosphatase) (MKP-L) (Mitogen-activated protein kinase phosphatase 6) (MAP kinase phosphatase 6) (MKP-6)
show all
Background:
dual specificity phosphatase 14(DUSP14) Homo sapiens Dual-specificity phosphatases (DUSPs) constitute a large heterogeneous subgroup of the type I cysteine-based protein-tyrosine phosphatase superfamily. DUSPs are characterized by their ability to dephosphorylate both tyrosine and serine/threonine residues. They have been implicated as major modulators of critical signaling pathways. DUSP14 contains the consensus DUSP C-terminal catalytic domain but lacks the N-terminal CH2 domain found in the MKP (mitogen-activated protein kinase phosphatase) class of DUSPs (see MIM 600714) (summary by Patterson et al., 2009 [PubMed 19228121]).[supplied by OMIM, Dec 2009],
show all
Function:
Catalytic activity:A phosphoprotein + H(2)O = a protein + phosphate.,Catalytic activity:Protein tyrosine phosphate + H(2)O = protein tyrosine + phosphate.,Function:Involved in the inactivation of MAP kinases. Dephosphorylates ERK, JNK and p38 MAP-kinases.,similarity:Belongs to the protein-tyrosine phosphatase family. Non-receptor class dual specificity subfamily.,similarity:Contains 1 tyrosine-protein phosphatase domain.,subunit:Interacts with CD28.,
show all
Tissue Expression:
Lung,
show all
Reference Citation({{totalcount}})
Catalog: YN0086
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$150.00
In stock
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}