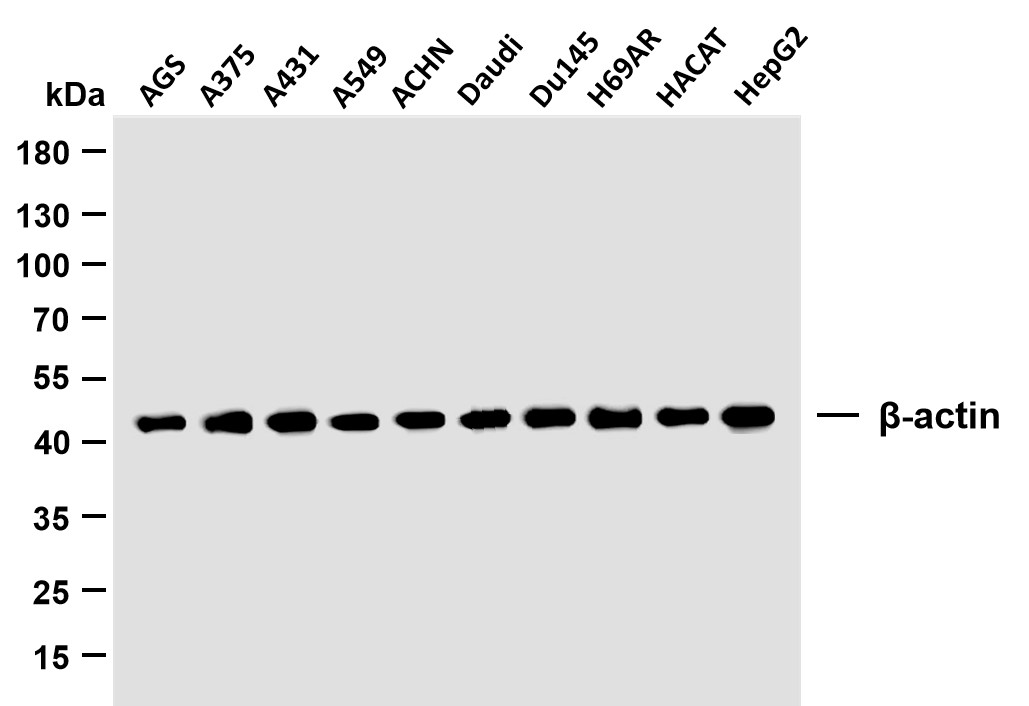
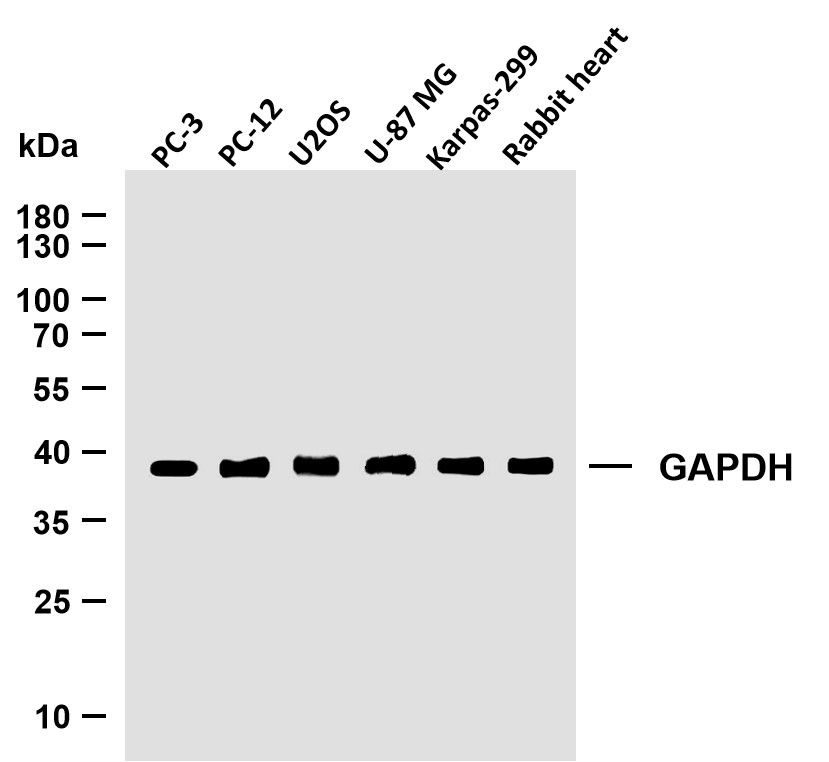
Catalog: YT7516
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$150.00
In stock
0
Add to cart


Collected


Collect
Main Information
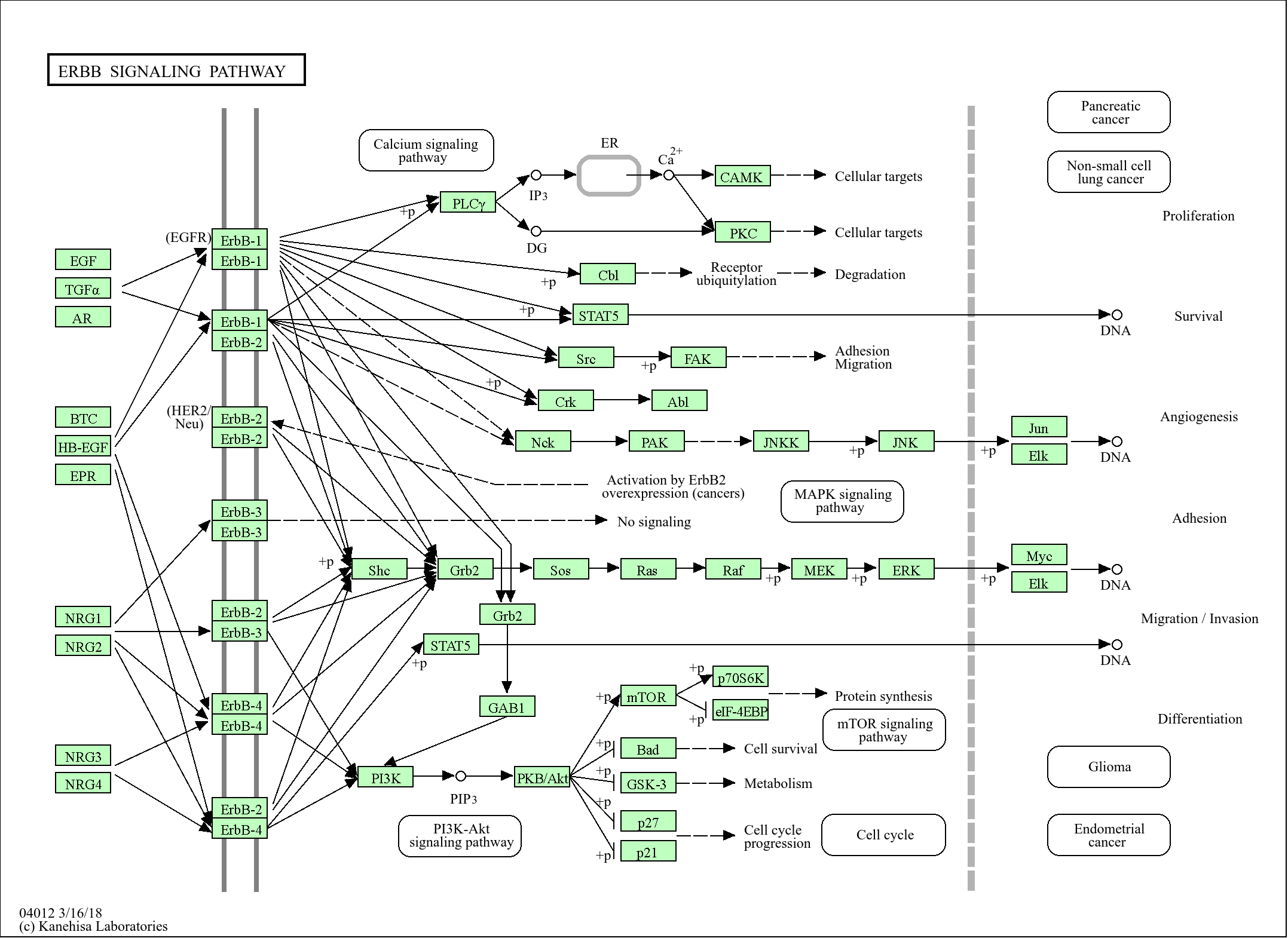
Target
ANM7
Host Species
Rabbit
Reactivity
Human, Mouse, Rat
Applications
WB
MW
76kD (Calculated)
Conjugate/Modification
Unmodified
Detailed Information
Recommended Dilution Ratio
WB 1:500-2000
Formulation
Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
Specificity
This antibody detects endogenous levels of ANM7 at Human/Mouse/Rat
Purification
The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
Storage
-15°C to -25°C/1 year(Do not lower than -25°C)
Concentration
1 mg/ml
MW(Calculated)
76kD
Modification
Unmodified
Clonality
Polyclonal
Isotype
IgG
Related Products
Antigen&Target Information
Immunogen:
Synthesized peptide derived from human ANM7 AA range: 120-170
show all
Specificity:
This antibody detects endogenous levels of ANM7 at Human/Mouse/Rat
show all
Gene Name:
PRMT7 KIAA1933
show all
Protein Name:
ANM7
show all
Database Link:
Background:
Arginine methylation is an apparently irreversible protein modification catalyzed by arginine methyltransferases, such as PMT7, using S-adenosylmethionine (AdoMet) as the methyl donor. Arginine methylation is implicated in signal transduction, RNA transport, and RNA splicing (Miranda et al., 2004 [PubMed 15044439]).[supplied by OMIM, Mar 2008],
show all
Function:
Catalytic activity:S-adenosyl-L-methionine + [myelin basic protein]-arginine = S-adenosyl-L-homocysteine + [myelin basic protein]-N(omega)-methyl-arginine.,Catalytic activity:S-adenosyl-L-methionine + histone-arginine = S-adenosyl-L-homocysteine + histone-N(omega)-methyl-arginine.,Function:Arginine methyltransferase that can both catalyze the formation of omega-N monomethylarginine (MMA) and symmetrical dimethylarginine (sDMA), with a preference for the formation of MMA. Specifically mediates the symmetrical dimethylation of arginine residues in the small nuclear ribonucleoproteins Sm D1 (SNRPD1) and Sm D3 (SNRPD3); such methylation being required for the assembly and biogenesis of snRNP core particles. Specifically mediates the symmetric dimethylation of histone H4 'Arg-3' to form H4R3sme2. Plays a role in gene imprinting by being recruited by CTCFL at the H19 imprinted control region (ICR) and methylating histone H4 to form H4R3sme2, possibly leading to recruit DNA methyltransferases at these sites. May also play a role in embryonic stem cell (ESC) pluripotency. Also able to mediate the arginine methylation of histone H2A and myelin basic protein (MBP) in vitro; the relevance of such results is however unclear in vivo.,miscellaneous:May be involved in etoposide-induced cytotoxicity, a chemotherapeutic agent frequently used for testicular cancer and small-cell lung cancer that can cause cytotoxicity in the treatment of other cancers. Down-regulation confers increased sensitivity to the Top1 inhibitor camptothecin (CPT).,similarity:Belongs to the protein arginine N-methyltransferase family. PRMT7 subfamily.,subunit:Homodimer and heterodimer (By similarity). Interacts with CTCFL (By similarity). Interacts with PRMT5 and SNRPD3.,
show all
Cellular Localization:
Cytoplasm, cytosol . Nucleus .
show all
Reference Citation({{totalcount}})
Catalog: YT7516
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$150.00
In stock
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}