Catalog: YT4707
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$150.00
In stock
0
Add to cart


Collected


Collect
Main Information
Target
tPA
Host Species
Rabbit
Reactivity
Human, Mouse, Rat
Applications
WB, ELISA
MW
63kD (Observed)
Conjugate/Modification
Unmodified
Detailed Information
Recommended Dilution Ratio
WB 1:500-1:2000; ELISA 1:10000; Not yet tested in other applications.
Formulation
Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
Specificity
tPA Polyclonal Antibody detects endogenous levels of tPA protein.
Purification
The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
Storage
-15°C to -25°C/1 year(Do not lower than -25°C)
Concentration
1 mg/ml
MW(Observed)
63kD
Modification
Unmodified
Clonality
Polyclonal
Isotype
IgG
Related Products
Antigen&Target Information
Immunogen:
The antiserum was produced against synthesized peptide derived from human tPA. AA range:38-87
show all
Specificity:
tPA Polyclonal Antibody detects endogenous levels of tPA protein.
show all
Gene Name:
PLAT
show all
Protein Name:
Tissue-type plasminogen activator
show all
Other Name:
PLAT ;
Tissue-type plasminogen activator ;
t-PA ;
t-plasminogen activator ;
tPA ;
Alteplase ;
Reteplase
Tissue-type plasminogen activator ;
t-PA ;
t-plasminogen activator ;
tPA ;
Alteplase ;
Reteplase
show all
Database Link:
Background:
This gene encodes tissue-type plasminogen activator, a secreted serine protease that converts the proenzyme plasminogen to plasmin, a fibrinolytic enzyme. The encoded preproprotein is proteolytically processed by plasmin or trypsin to generate heavy and light chains. These chains associate via disulfide linkages to form the heterodimeric enzyme. This enzyme plays a role in cell migration and tissue remodeling. Increased enzymatic activity causes hyperfibrinolysis, which manifests as excessive bleeding, while decreased activity leads to hypofibrinolysis, which can result in thrombosis or embolism. Alternative splicing of this gene results in multiple transcript variants, at least one of which encodes an isoform that is proteolytically processed. [provided by RefSeq, Jan 2016],
show all
Function:
Catalytic activity:Specific cleavage of Arg-|-Val bond in plasminogen to form plasmin.,Disease:Increased activity of TPA is the cause of hyperfibrinolysis [MIM:173370]. Hyperfibrinolysis leads to excessive bleeding. Defective release of TPA causes hypofibrinolysis, leading to thrombosis or embolism.,Domain:Both FN1 and EGF-like domains are important for binding to LRP1.,Domain:Both FN1 and one of the kringle domains are required for binding to fibrin.,Domain:The FN1 domain mediates binding to annexin A2.,Domain:The second kringle domain is implicated in binding to cytokeratin-8 and to the endothelial cell surface binding site.,Function:Converts the abundant, but inactive, zymogen plasminogen to plasmin by hydrolyzing a single Arg-Val bond in plasminogen. By controlling plasmin-mediated proteolysis, it plays an important role in tissue remodeling and degradation, in cell migration and many other physiopathological events. Play a direct role in facilitating neuronal migration.,online information:Clinical information on Activase,online information:Clinical information on Retavase,online information:The Singapore human mutation and polymorphism database,online information:Tissue plasminogen activator entry,pharmaceutical:Available under the names Activase (Genentech) and Retavase (Centocor and Roche) [Retavase is a fragment of TPA that contains kringle 2 and the protease domain; it was also known as BM 06.022]. Used in Acute Myocardial Infarction (AMI), in Acute Ischemic Stroke (AIS) and Pulmonary Embolism (PE) to initiate fibrinolysis.,PTM:Characterization of O-linked glycan was studied in Bowes melanoma cell line.,PTM:Differential cell-specific N-linked glycosylation gives rise to two glycoforms, type I (glycosylated at Asn-219) and type II (not glycosylated at Asn-219). The single chain type I glycoform is less readily converted into the two-chain form by plasmin, and the two-chain type I glycoform has a lower activity than the two-chain type II glycoform in the presence of fibrin.,PTM:N-glycosylation of Asn-152; the bound oligomannosidic glycan is involved in the interaction with the mannose receptor.,PTM:The single chain, almost fully active enzyme, can be further processed into a two-chain fully active form by a cleavage after Arg-310 catalyzed by plasmin, tissue kallikrein or factor Xa.,similarity:Belongs to the peptidase S1 family.,similarity:Contains 1 EGF-like domain.,similarity:Contains 1 fibronectin type-I domain.,similarity:Contains 1 peptidase S1 domain.,similarity:Contains 2 kringle domains.,subunit:Heterodimer of chain A and chain B held by a disulfide bond. Binds to fibrin with high affinity. This interaction leads to an increase in the catalytic efficiency of the enzyme between 100-fold and 1000-fold, due to an increase in affinity for plasminogen. Similarly, binding to heparin increases the activation of plasminogen. Binds to annexin A2, cytokeratin-8, fibronectin and laminin. Binds to mannose receptor and the low-density lipoprotein receptor-related protein (LRP1); these proteins are involved in TPA clearance. Yet unidentified interactions on endothelial cells and vascular smooth muscle cells (VSMC) lead to a 100-fold stimulation of plasminogen activation. In addition, binding to VSMC reduces TPA inhibition by PAI-1 by 30-fold. Binds LRP1B; binding is followed by internalization and degradation.,tissue specificity:Synthesized in numerous tissues (including tumors) and secreted into most extracellular body fluids, such as plasma, uterine fluid, saliva, gingival crevicular fluid, tears, seminal fluid, and milk.,
show all
Cellular Localization:
Secreted, extracellular space.
show all
Tissue Expression:
Synthesized in numerous tissues (including tumors) and secreted into most extracellular body fluids, such as plasma, uterine fluid, saliva, gingival crevicular fluid, tears, seminal fluid, and milk.
show all
Research Areas:
>>Apelin signaling pathway ;
>>Complement and coagulation cascades ;
>>Transcriptional misregulation in cancer ;
>>Prostate cancer ;
>>Fluid shear stress and atherosclerosis
>>Complement and coagulation cascades ;
>>Transcriptional misregulation in cancer ;
>>Prostate cancer ;
>>Fluid shear stress and atherosclerosis
show all
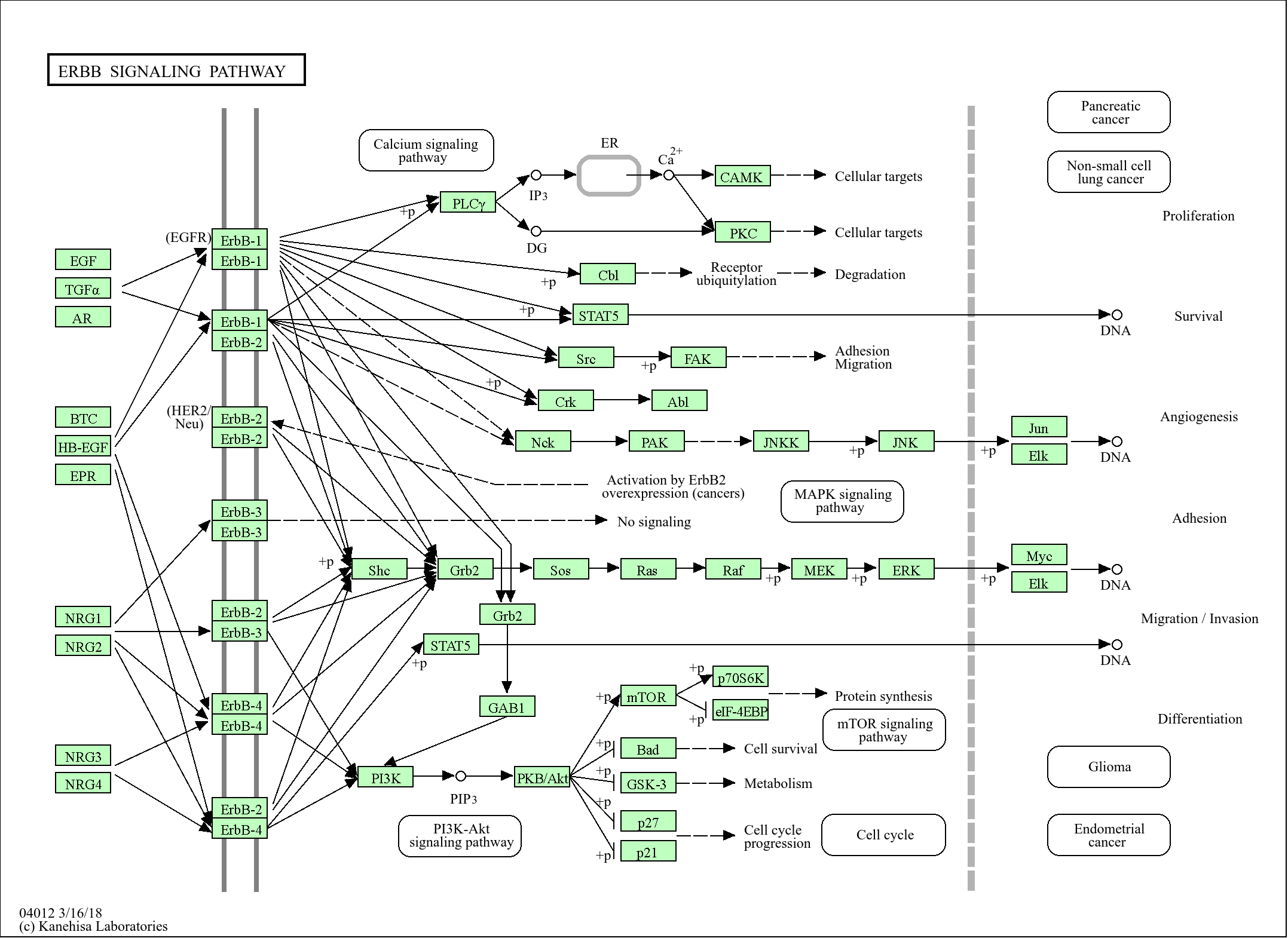
Signaling Pathway
Organismal Systems >> Immune system >> Complement and coagulation cascades
Human Diseases >> Cancer: overview >> Transcriptional misregulation in cancer
Human Diseases >> Cancer: specific types >> Prostate cancer
Environmental Information Processing >> Signal transduction >> Apelin signaling pathway
Reference Citation({{totalcount}})
Catalog: YT4707
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$150.00
In stock
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}