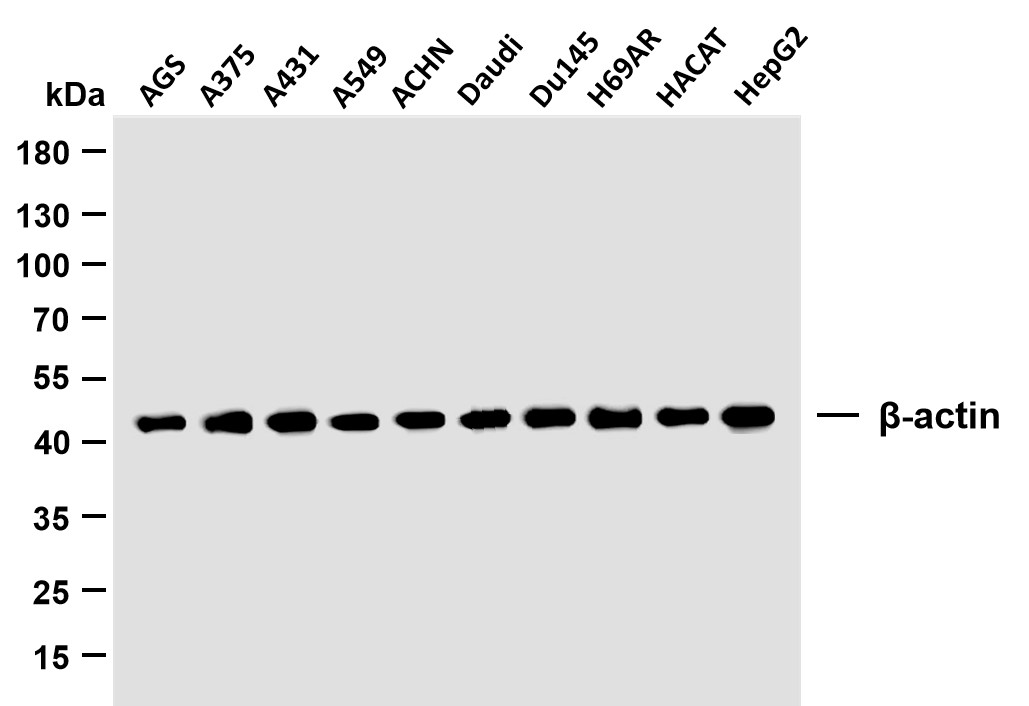
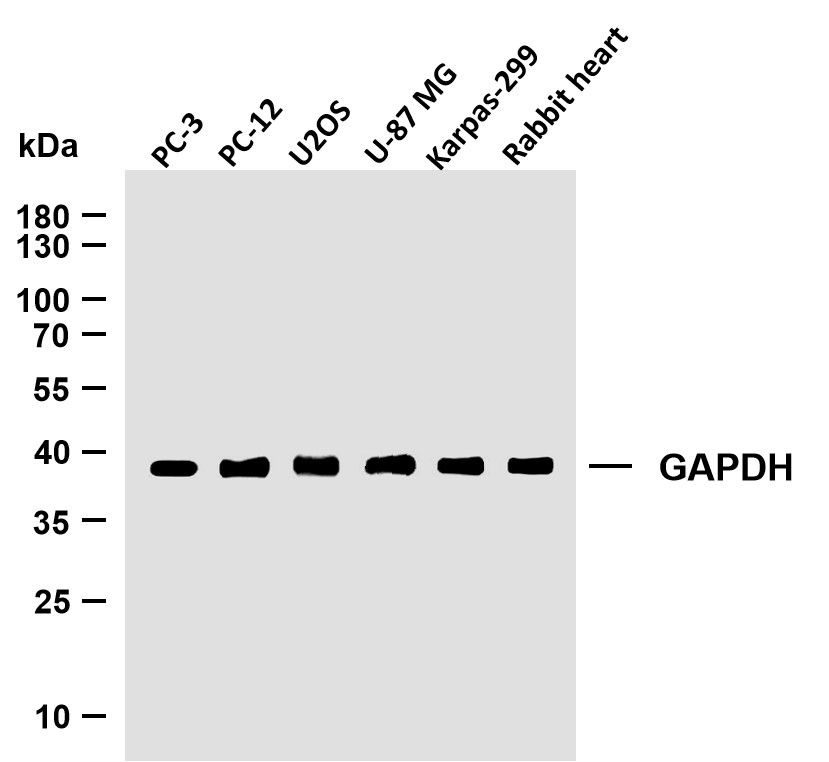
Catalog: YN7679
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$160.00
In stock
0
Add to cart


Collected


Collect
Main Information
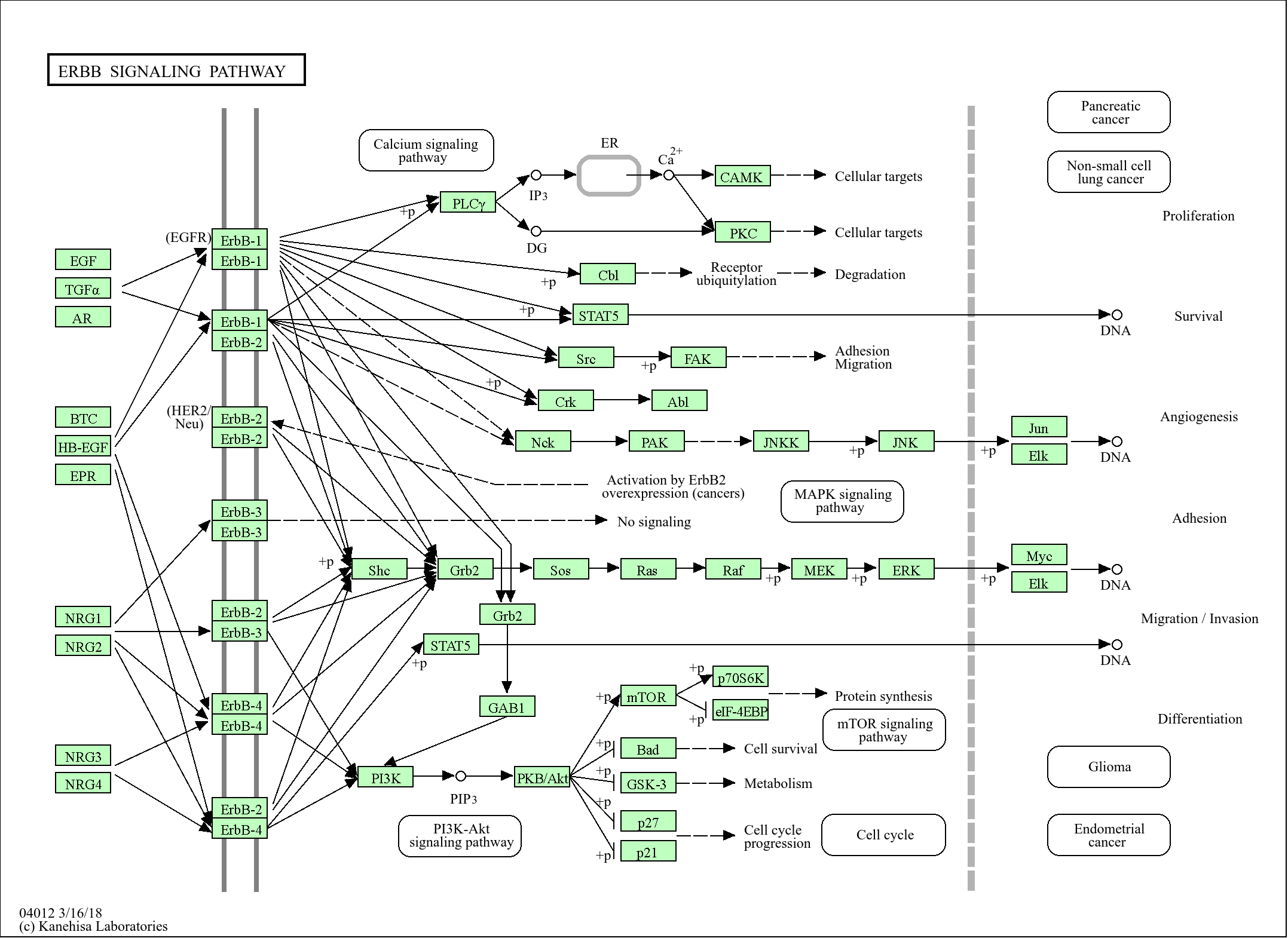
Target
BRCC3
Host Species
Rabbit
Reactivity
Human, Mouse, Rat
Applications
WB
MW
35kD (Calculated)
Conjugate/Modification
Unmodified
Detailed Information
Recommended Dilution Ratio
WB 1:500-2000
Formulation
Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
Specificity
This antibody detects endogenous levels of BRCC3 at Human, Mouse,Rat
Purification
The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
Storage
-15°C to -25°C/1 year(Do not lower than -25°C)
Concentration
1 mg/ml
MW(Calculated)
35kD
Modification
Unmodified
Clonality
Polyclonal
Isotype
IgG
Related Products
Antigen&Target Information
Immunogen:
Synthesized peptide derived from human BRCC3
show all
Specificity:
This antibody detects endogenous levels of BRCC3 at Human, Mouse,Rat
show all
Gene Name:
BRCC3 BRCC36 C6.1A CXorf53
show all
Protein Name:
Lys-63-specific deubiquitinase BRCC36 (BRCA1-A complex subunit BRCC36) (BRCA1/BRCA2-containing complex subunit 3) (BRCA1/BRCA2-containing complex subunit 36) (BRISC complex subunit BRCC36)
show all
Database Link:
Function:
Metalloprotease that specifically cleaves 'Lys-63'-linked polyubiquitin chains . Does not have activity toward 'Lys-48'-linked polyubiquitin chains. Component of the BRCA1-A complex, a complex that specifically recognizes 'Lys-63'-linked ubiquitinated histones H2A and H2AX at DNA lesions sites, leading to target the BRCA1-BARD1 heterodimer to sites of DNA damage at double-strand breaks (DSBs). In the BRCA1-A complex, it specifically removes 'Lys-63'-linked ubiquitin on histones H2A and H2AX, antagonizing the RNF8-dependent ubiquitination at double-strand breaks (DSBs) . Catalytic subunit of the BRISC complex, a multiprotein complex that specifically cleaves 'Lys-63'-linked ubiquitin in various substrates . Mediates the specific 'Lys-63'-specific deubiquitination associated with the COP9 signalosome complex (CSN), via the interaction of the BRISC complex with the CSN complex . The BRISC complex is required for normal mitotic spindle assembly and microtubule attachment to kinetochores via its role in deubiquitinating NUMA1 . Plays a role in interferon signaling via its role in the deubiquitination of the interferon receptor IFNAR1; deubiquitination increases IFNAR1 activity by enhancing its stability and cell surface expression . Down-regulates the response to bacterial lipopolysaccharide (LPS) via its role in IFNAR1 deubiquitination . Deubiquitinates HDAC1 and PWWP2B leading to their stabilization (By similarity).
show all
Cellular Localization:
Nucleus . Cytoplasm . Cytoplasm, cytoskeleton, spindle pole . Localizes at sites of DNA damage at double-strand breaks (DSBs) (PubMed:20656690, PubMed:26344097). Interaction with ABRAXAS2 retains BRCC3 in the cytoplasm (PubMed:20656690). .
show all
Tissue Expression:
Heart, brain, placenta, lung, liver, skeletal muscle, kidney and pancreas. Aberrantly expressed in the vast majority of breast tumors.
show all
Reference Citation({{totalcount}})
Catalog: YN7679
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$160.00
In stock
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}