Catalog: YN4146
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$150.00
In stock
0
Add to cart


Collected


Collect
Main Information
Target
DYH5
Host Species
Rabbit
Reactivity
Human, Mouse
Applications
IHC, IF
MW
509kD (Calculated)
Conjugate/Modification
Unmodified
Detailed Information
Recommended Dilution Ratio
IHC 1:50-200; IF 1:50-200
Formulation
Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
Specificity
This antibody detects endogenous levels of DYH5 at Human/Mouse
Purification
The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
Storage
-15°C to -25°C/1 year(Do not lower than -25°C)
Concentration
1 mg/ml
MW(Calculated)
509kD
Modification
Unmodified
Clonality
Polyclonal
Isotype
IgG
Related Products
Antigen&Target Information
Immunogen:
Synthesized peptide derived from human DYH5 AA range: 2445-2495
show all
Specificity:
This antibody detects endogenous levels of DYH5 at Human/Mouse
show all
Gene Name:
DNAH5 DNAHC5 HL1 KIAA1603
show all
Protein Name:
DYH5
show all
Background:
This gene encodes a dynein protein, which is part of a microtubule-associated motor protein complex consisting of heavy, light, and intermediate chains. This protein is an axonemal heavy chain dynein. It functions as a force-generating protein with ATPase activity, whereby the release of ADP is thought to produce the force-producing power stroke. Mutations in this gene cause primary ciliary dyskinesia type 3, as well as Kartagener syndrome, which are both diseases due to ciliary defects. [provided by RefSeq, Oct 2009],
show all
Function:
Disease:Defects in DNAH5 are a cause of Kartagener syndrome (KTGS) [MIM:244400]. KTGS is an autosomal recessive disorder characterized by the association of primary ciliary dyskinesia with situs inversus. Clinical features include recurrent respiratory infections, bronchiectasis, infertility, and lateral transposition of the viscera of the thorax and abdomen. The situs inversus is most often total, although it can be partial in some cases (isolated dextrocardia or isolated transposition of abdominal viscera).,Disease:Defects in DNAH5 are the cause of primary ciliary dyskinesia type 3 (CILD3) [MIM:608644]. CILD3 is an autosomal recessive disorder characterized by axonemal abnormalities of motile cilia. Respiratory infections leading to chronic inflammation and bronchiectasis are recurrent, due to defects in the respiratory cilia; reduced fertility is often observed in male patients due to abnormalities of sperm tails. Half of the patients exhibit situs inversus, due to dysfunction of monocilia at the embryonic node and randomization of left-right body asymmetry. Primary ciliary dyskinesia associated with situs inversus is referred to as Kartagener syndrome.,Domain:Dynein heavy chains probably consist of an N-terminal stem (which binds cargo and interacts with other dynein components), and the head or motor domain. The motor contains six tandemly-linked AAA domains in the head, which form a ring. A stalk-like structure (formed by two of the coiled coil domains) protrudes between AAA 4 and AAA 5 and terminates in a microtubule-binding site. A seventh domain may also contribute to this ring; it is not clear whether the N-terminus or the C-terminus forms this extra domain. There are four well-conserved and two non-conserved ATPase sites, one per AAA domain. Probably only one of these (within AAA 1) actually hydrolyzes ATP, the others may serve a regulatory function.,Function:Force generating protein of respiratory cilia. Produces force towards the minus ends of microtubules. Dynein has ATPase activity; the force-producing power stroke is thought to occur on release of ADP. Required for structural and functional integrity of the cilia of ependymal cells lining the brain ventricles.,similarity:Belongs to the dynein heavy chain family.,subunit:Consists of at least two heavy chains and a number of intermediate and light chains.,
show all
Cellular Localization:
Cytoplasm, cytoskeleton, cilium axoneme .
show all
Tissue Expression:
Expressed in airway epithelial cells (at protein level). Not detected in spermatozoa (at protein level).
show all
Research Areas:
>>Amyotrophic lateral sclerosis ;
>>Huntington disease ;
>>Pathways of neurodegeneration - multiple diseases
>>Huntington disease ;
>>Pathways of neurodegeneration - multiple diseases
show all
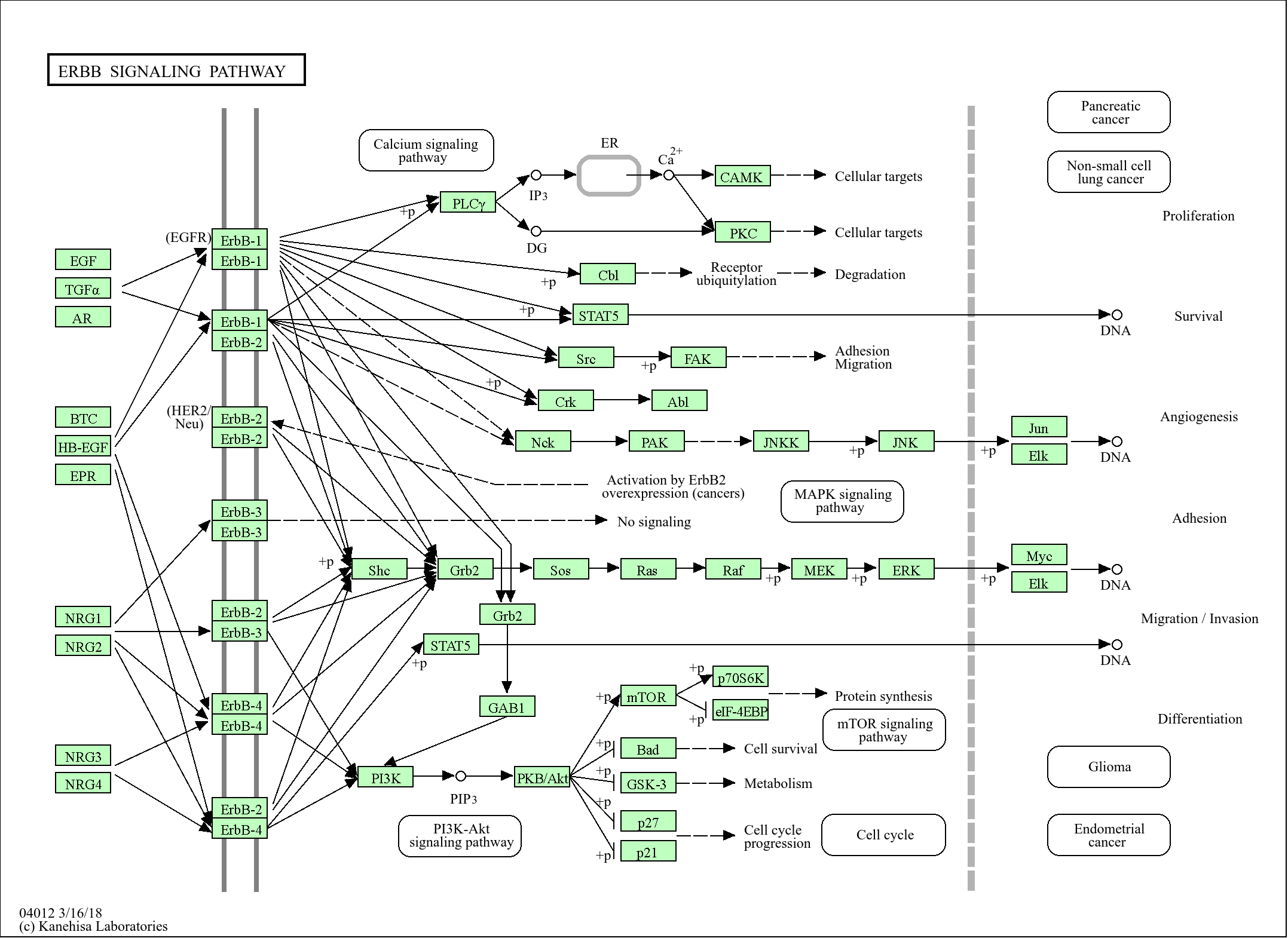
Signaling Pathway
Reference Citation({{totalcount}})
Catalog: YN4146
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$150.00
In stock
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}