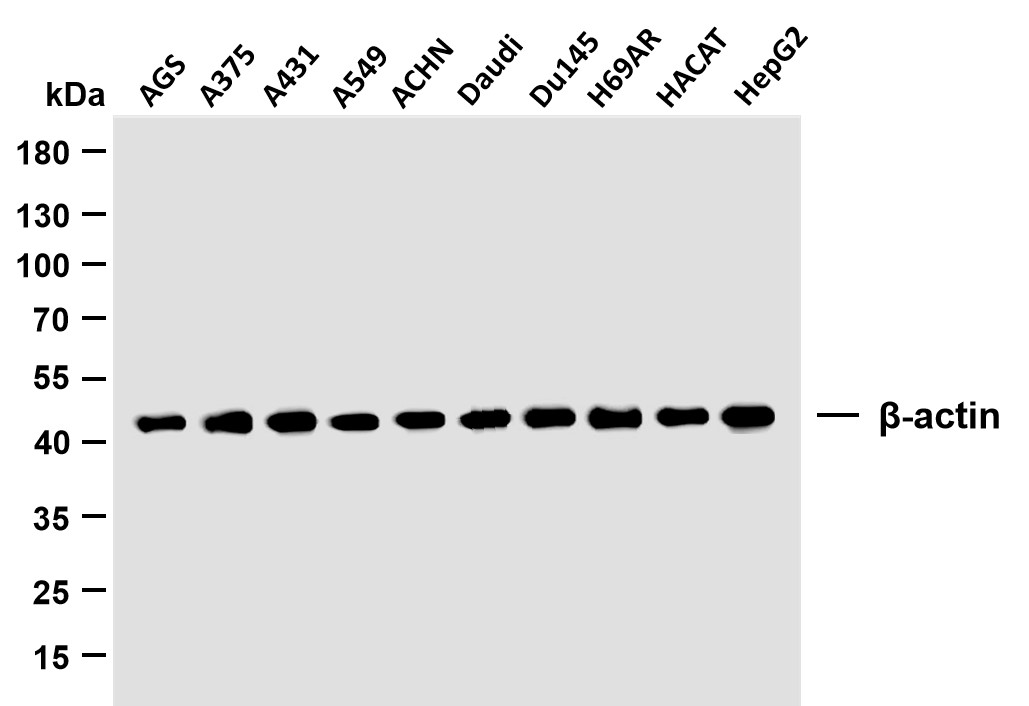
Catalog: YN2215
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$150.00
In stock
0
Add to cart


Collected


Collect
Main Information
Target
ACY1
Host Species
Rabbit
Reactivity
Human, Mouse
Applications
WB, ELISA
MW
44kD (Observed)
Conjugate/Modification
Unmodified
Detailed Information
Recommended Dilution Ratio
WB 1:500-2000; ELISA 1:5000-20000
Formulation
Liquid in PBS containing 50% glycerol,0.5% BSA and 0.02% sodium azide.
Specificity
ACY1 Polyclonal Antibody detects endogenous levels of protein.
Purification
The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
Storage
-15°C to -25°C/1 year(Do not lower than -25°C)
Concentration
1 mg/ml
MW(Observed)
44kD
Modification
Unmodified
Clonality
Polyclonal
Isotype
IgG
Related Products
Antigen&Target Information
Immunogen:
Synthesized peptide derived from part region of human protein
show all
Specificity:
ACY1 Polyclonal Antibody detects endogenous levels of protein.
show all
Gene Name:
ACY1
show all
Protein Name:
Aminoacylase-1 (ACY-1) (N-acyl-L-amino-acid amidohydrolase)
show all
Background:
This gene encodes a cytosolic, homodimeric, zinc-binding enzyme that catalyzes the hydrolysis of acylated L-amino acids to L-amino acids and an acyl group, and has been postulated to function in the catabolism and salvage of acylated amino acids. This gene is located on chromosome 3p21.1, a region reduced to homozygosity in small-cell lung cancer (SCLC), and its expression has been reported to be reduced or undetectable in SCLC cell lines and tumors. The amino acid sequence of human aminoacylase-1 is highly homologous to the porcine counterpart, and this enzyme is the first member of a new family of zinc-binding enzymes. Mutations in this gene cause aminoacylase-1 deficiency, a metabolic disorder characterized by central nervous system defects and increased urinary excretion of N-acetylated amino acids. Alternative splicing of this gene results in multiple transcript variants. Read-through transcription als
show all
Function:
Catalytic activity:An N-acyl-L-amino acid + H(2)O = a carboxylate + an L-amino acid.,cofactor:Binds 2 zinc ions per subunit.,Disease:Defects in ACY1 are the cause of aminoacylase-1 deficiency (ACY1D) [MIM:609924]. ACY1D results in a metabolic disorder manifesting with encephalopathy, unspecific psychomotor delay, psychomotor delay with atrophy of the vermis and syringomyelia, marked muscular hypotonia or normal clinical features. Epileptic seizures are a frequent feature. All affected individuals exhibit markedly increased urinary excretion of several N-acetylated amino acids.,Function:Involved in the hydrolysis of N-acylated or N-acetylated amino acids (except L-aspartate).,similarity:Belongs to the peptidase M20A family.,subunit:Homodimer. Interacts with SPHK1.,tissue specificity:Expression is highest in kidney, strong in brain and weaker in placenta and spleen.,
show all
Cellular Localization:
Cytoplasm.
show all
Tissue Expression:
Research Areas:
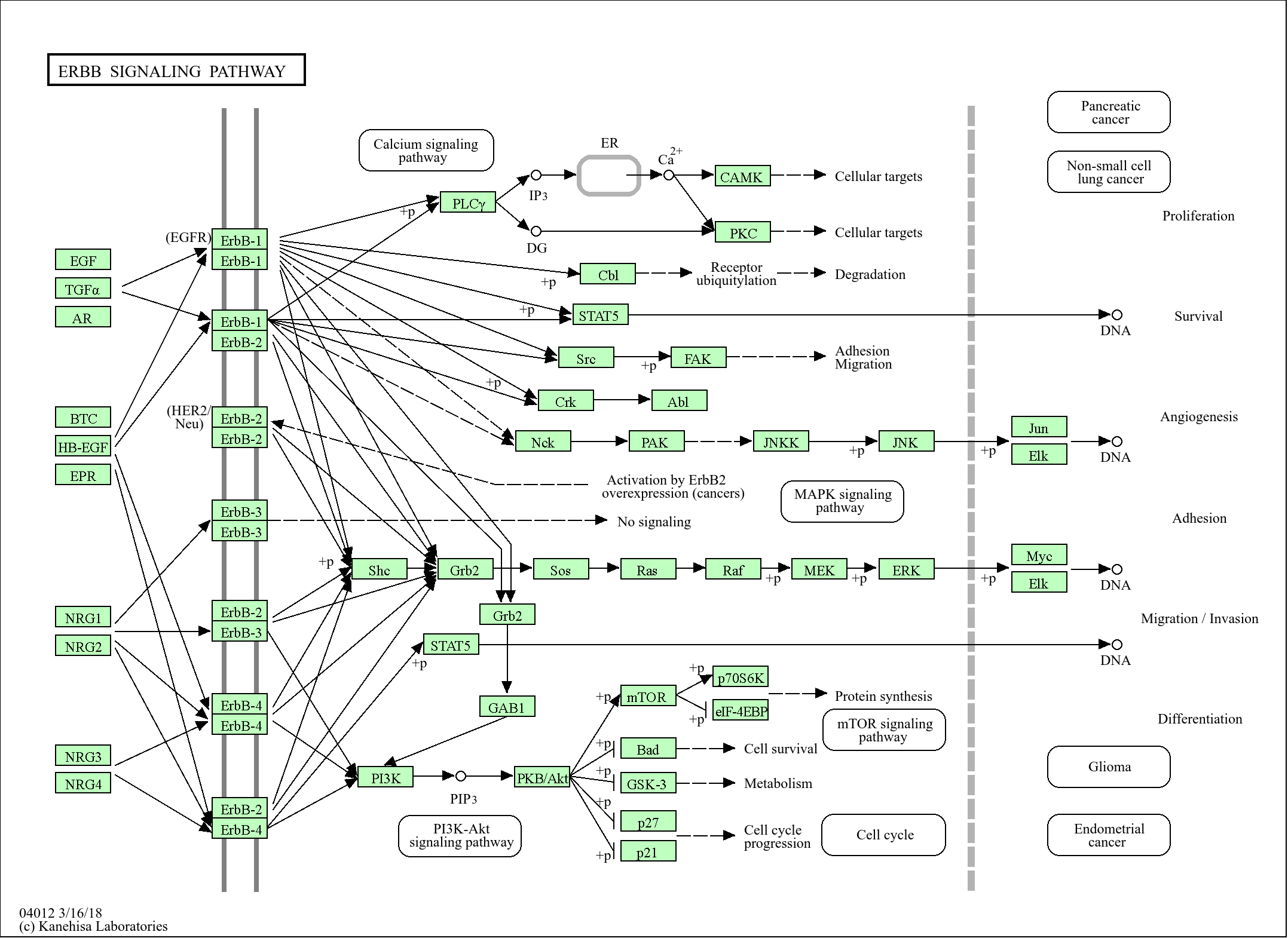
>>Arginine biosynthesis ;
>>Metabolic pathways ;
>>2-Oxocarboxylic acid metabolism ;
>>Biosynthesis of amino acids
>>Metabolic pathways ;
>>2-Oxocarboxylic acid metabolism ;
>>Biosynthesis of amino acids
show all
Reference Citation({{totalcount}})
Catalog: YN2215
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$150.00
In stock
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}