Catalog: YM0550
Size
Price
Status
Qty.
200μL
$600.00
3 weeks
0
100μL
$350.00
3 weeks
0
50μL
$210.00
3 weeks
0
Add to cart


Collected


Collect
Main Information
Target
RAG-2
Host Species
Mouse
Reactivity
Human
Applications
WB, ELISA
MW
59kD (Calculated)
Conjugate/Modification
Unmodified
Detailed Information
Recommended Dilution Ratio
WB 1:500-1:2000; ELISA 1:10000; Not yet tested in other applications.
Formulation
Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
Specificity
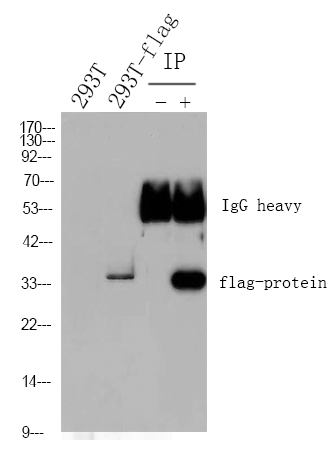
RAG-2 Monoclonal Antibody detects endogenous levels of RAG-2 protein.
Purification
Affinity purification
Storage
-15°C to -25°C/1 year(Do not lower than -25°C)
MW(Calculated)
59kD
Modification
Unmodified
Clonality
Monoclonal
Related Products
Antigen&Target Information
Immunogen:
Purified recombinant fragment of human RAG-2 (350-527aa) expressed in E. Coli.
show all
Specificity:
RAG-2 Monoclonal Antibody detects endogenous levels of RAG-2 protein.
show all
Gene Name:
RAG2
show all
Protein Name:
V(D)J recombination-activating protein 2
show all
Other Name:
RAG2 ;
V ;
D ;
J recombination-activating protein 2 ;
RAG-2
V ;
D ;
J recombination-activating protein 2 ;
RAG-2
show all
Background:
This gene encodes a protein that is involved in the initiation of V(D)J recombination during B and T cell development. This protein forms a complex with the product of the adjacent recombination activating gene 1, and this complex can form double-strand breaks by cleaving DNA at conserved recombination signal sequences. The recombination activating gene 1 component is thought to contain most of the catalytic activity, while the N-terminal of the recombination activating gene 2 component is thought to form a six-bladed propeller in the active core that serves as a binding scaffold for the tight association of the complex with DNA. A C-terminal plant homeodomain finger-like motif in this protein is necessary for interactions with chromatin components, specifically with histone H3 that is trimethylated at lysine 4. Mutations in this gene cause Omenn syndrome, a form of severe combined immunodef
show all
Function:
Disease:Defects in RAG2 are a cause of combined cellular and humoral immune defects with granulomas (CHIDG) [MIM:233650]. CHIDG is an immunodeficiency disease with granulomas in the skin, mucous membranes, and internal organs. Other characteristics include hypogammaglobulinemia, a diminished number of T and B cells, and sparse thymic tissue on ultrasonography.,Disease:Defects in RAG2 are a cause of Omenn syndrome (OS) [MIM:603554]; a severe immunodeficiency characterized by the presence of activated, anergic, oligoclonal T-cells, hypereosinophilia, and high IgE levels.,Disease:Defects in RAG2 are a cause of severe combined immunodeficiency, autosomal recessive T cell-negative, B-cell-negative, NK cell-positive (T(-)B(-)NK(+)SCID) [MIM:601457]. SCID refers to a genetically and clinically heterogeneous group of rare congenital disorders characterized by impairment of both humoral and cell-mediated immunity, leukopenia, and low or absent antibody levels. Patients with SCID present in infancy with recurrent, persistent infections by opportunistic organisms. The common characteristic of all types of SCID is absence of T-cell-mediated cellular immunity due to a defect in T-cell development.,Function:During lymphocyte development, the genes encoding immunoglobulins and T-cell receptors are assembled from variable (V), diversity (D), and joining (J) gene segments. This combinatorial process, known as V(D)J recombination, allows the generation of an enormous range of binding specificities from a limited amount of genetic information. The RAG1/RAG2 complex initiates this process by binding to the conserved recombination signal sequences (RSS) and introducing a double-strand break between the RSS and the adjacent coding segment. These breaks are generated in two steps, nicking of one strand (hydrolysis), followed by hairpin formation (transesterification). RAG1/2 has also been shown to function as a transposase in vitro, and to possess RSS-independent endonuclease activity (end processing) and hairpin opening. RAG1 alone can bind to RSS but stable, efficient binding requires RAG2. All known catalytic activities require the presence of both proteins.,online information:RAG2 deficiency database,similarity:Belongs to the RAG2 family.,subunit:The RAG complexes appear to contain three to five molecules of RAG2 for each molecule of RAG1.,tissue specificity:Cells of the B- and T-lymphocyte lineages.,
show all
Cellular Localization:
Nucleus .
show all
Tissue Expression:
Cells of the B- and T-lymphocyte lineages.
show all
Research Areas:
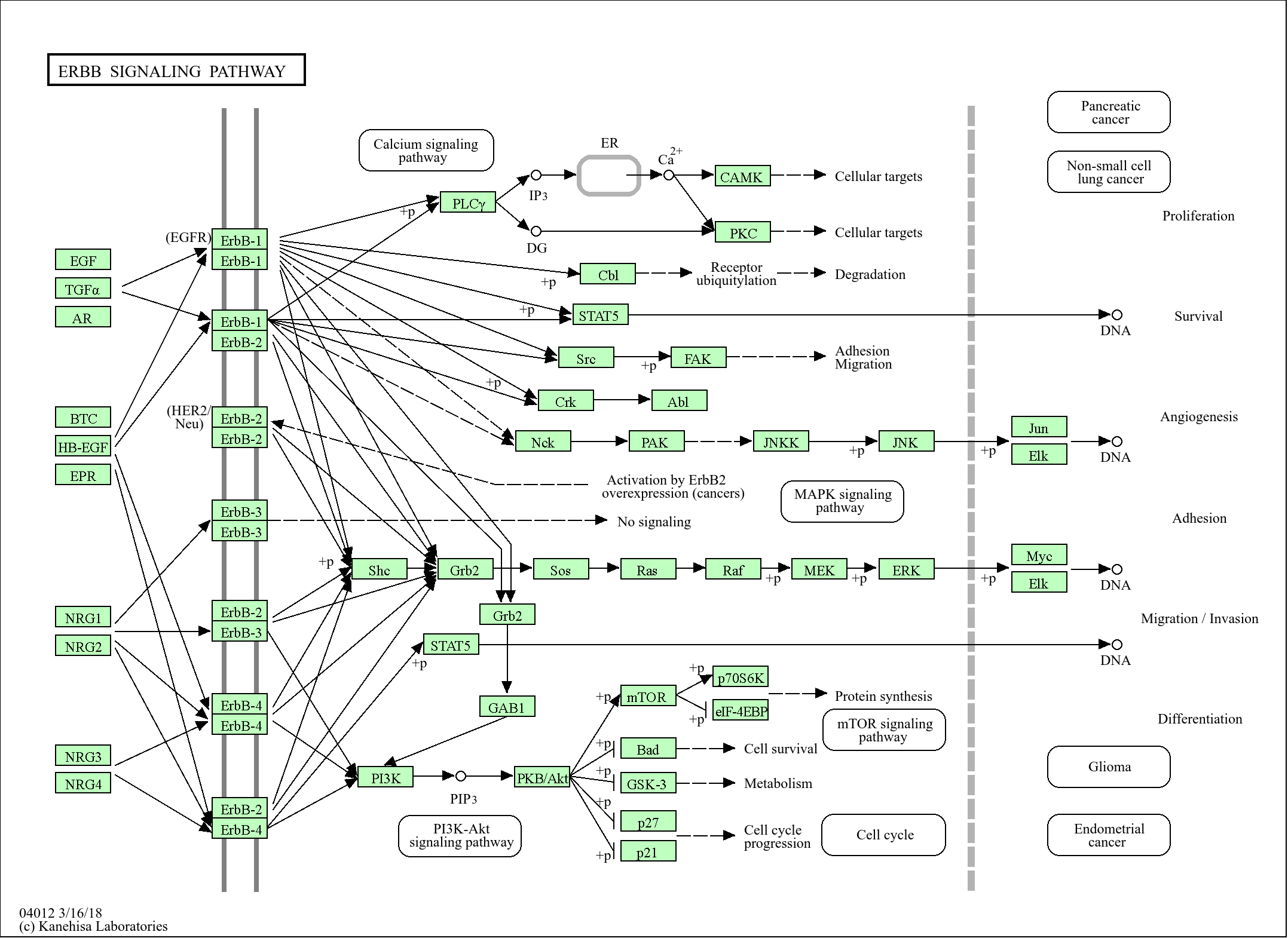
>>FoxO signaling pathway ;
>>Primary immunodeficiency
>>Primary immunodeficiency
show all
Signaling Pathway
Reference Citation({{totalcount}})
Catalog: YM0550
Size
Price
Status
Qty.
200μL
$600.00
3 weeks
0
100μL
$350.00
3 weeks
0
50μL
$210.00
3 weeks
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}