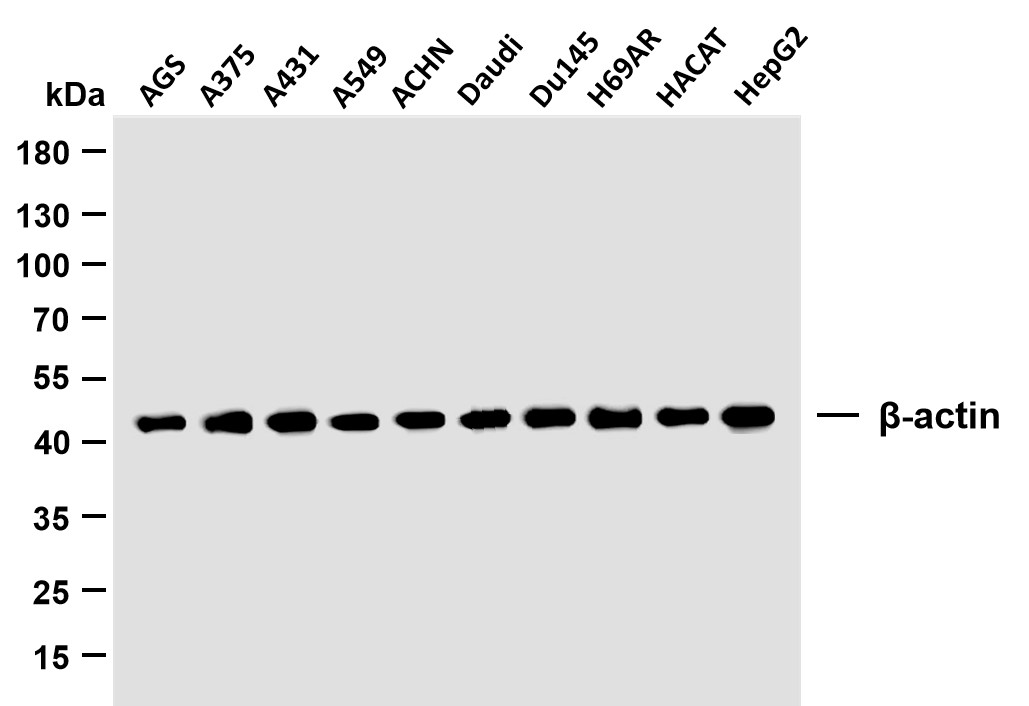
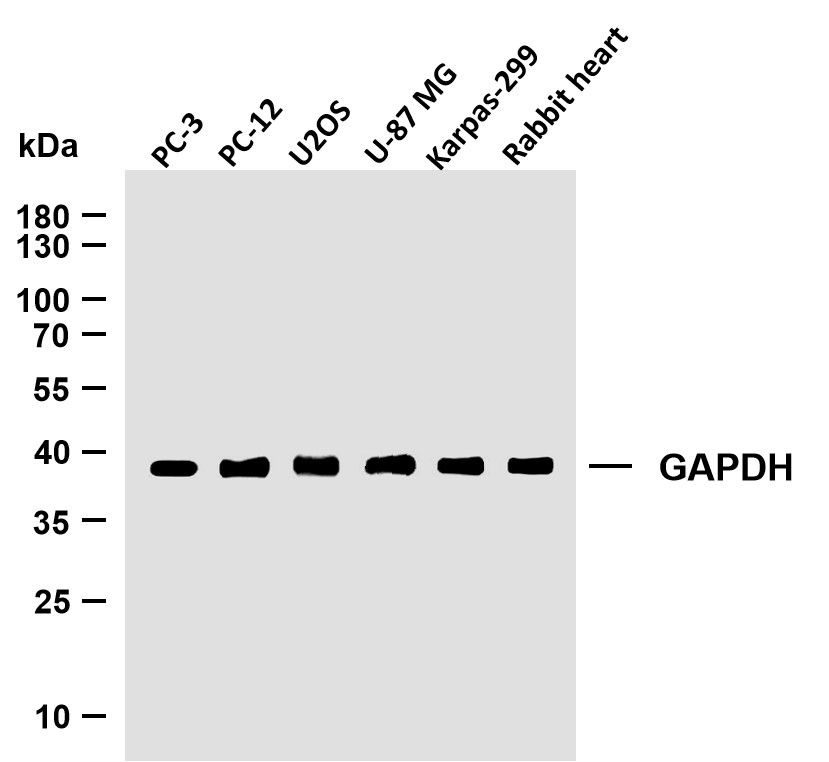
Catalog: YC0065
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$150.00
In stock
0
Add to cart


Collected


Collect
Main Information
Target
MMP-27
Host Species
Rabbit
Reactivity
Human, Monkey
Applications
WB, ELISA
MW
48kD (Observed)
Conjugate/Modification
Unmodified
Detailed Information
Recommended Dilution Ratio
WB 1:500-1:2000; ELISA 1:10000; Not yet tested in other applications.
Formulation
Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
Specificity
Cleaved-MMP-27 (Y99) Polyclonal Antibody detects endogenous levels of fragment of activated MMP-27 protein resulting from cleavage adjacent to Y99.
Purification
The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
Storage
-15°C to -25°C/1 year(Do not lower than -25°C)
Concentration
1 mg/ml
MW(Observed)
48kD
Modification
Unmodified
Clonality
Polyclonal
Isotype
IgG
Related Products
Antigen&Target Information
Immunogen:
The antiserum was produced against synthesized peptide derived from human MMP27. AA range:80-129
show all
Specificity:
Cleaved-MMP-27 (Y99) Polyclonal Antibody detects endogenous levels of fragment of activated MMP-27 protein resulting from cleavage adjacent to Y99.
show all
Gene Name:
MMP27
show all
Protein Name:
Matrix metalloproteinase-27
show all
Other Name:
MMP27 ;
Matrix metalloproteinase-27 ;
MMP-27
Matrix metalloproteinase-27 ;
MMP-27
show all
Background:
Proteins of the matrix metalloproteinase (MMP) family are involved in the breakdown of extracellular matrix in normal physiological processes, such as embryonic development, reproduction, and tissue remodeling, as well as in disease processes, such as arthritis and metastasis. Most MMP's are secreted as inactive proproteins which are activated when cleaved by extracellular proteinases. [provided by RefSeq, Jul 2008],
show all
Function:
cofactor:Binds 2 zinc ions per subunit.,cofactor:Binds 4 calcium ions per subunit.,Domain:The conserved cysteine present in the cysteine-switch motif binds the catalytic zinc ion, thus inhibiting the enzyme. The dissociation of the cysteine from the zinc ion upon the activation-peptide release activates the enzyme.,Function:Matrix metalloproteinases degrade protein components of the extracellular matrix such as fibronectin, laminin, gelatins and/or collagens.,similarity:Belongs to the peptidase M10A family.,similarity:Contains 4 hemopexin-like domains.,tissue specificity:Expressed in B-cells.,
show all
Cellular Localization:
Endoplasmic reticulum membrane; Peripheral membrane protein . Retained in the endoplasmic reticulum. .
show all
Tissue Expression:
Expressed in B-cells (PubMed:14506071). Expressed in a subset of endometrial macrophages related to menstruation and in ovarian and peritoneal endometriotic lesions (at protein level)(PubMed:24810263).
show all
Reference Citation({{totalcount}})
Catalog: YC0065
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$150.00
In stock
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}