Catalog: KA3976C
Size
Price
Status
Qty.
96well
$330.00
In stock
0
Add to cart


Collected


Collect
Main Information
Reactivity
Human, Mouse, Rat
Applications
ELISA
Conjugate/Modification
Unmodified
Detailed Information
Storage
2-8°C/6 months,Ship by ice bag
Modification
Unmodified
Detection Method
Colorimetric
Related Products
Antigen&Target Information
Gene Name:
SUMO2
show all
Other Name:
Small ubiquitin-related modifier 2 ;
SUMO-2 ;
HSMT3 ;
SMT3 homolog 2 ;
SUMO-3 ;
Sentrin-2 ;
Ubiquitin-like protein SMT3A ;
Smt3A ;
SUMO-2 ;
HSMT3 ;
SMT3 homolog 2 ;
SUMO-3 ;
Sentrin-2 ;
Ubiquitin-like protein SMT3A ;
Smt3A ;
show all
Background:
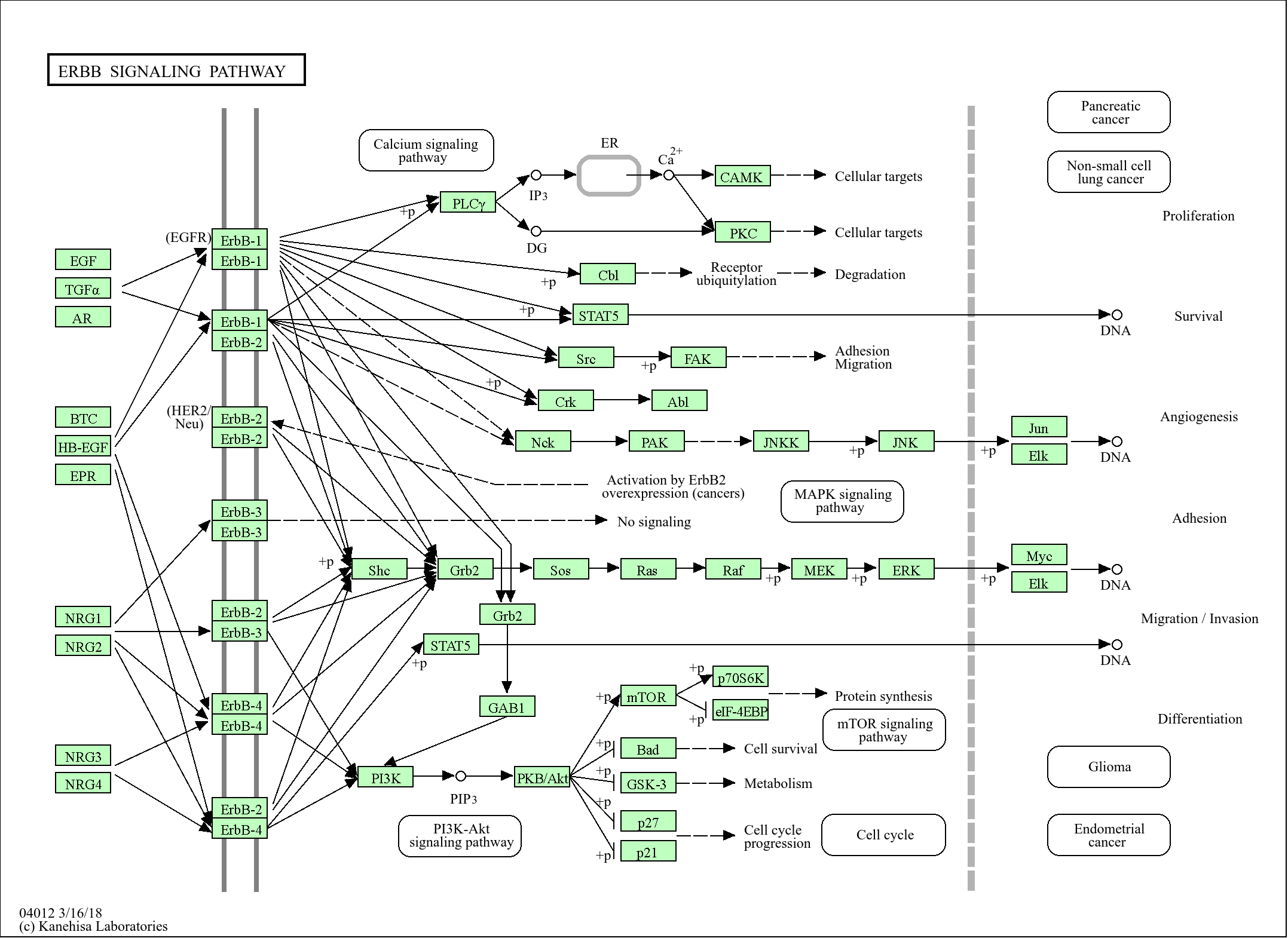
function:Ubiquitin-like protein which can be covalently attached to target lysines either as a monomer or as a lysine-linked polymer. Does not seem to be involved in protein degradation and may function as an antagonist of ubiquitin in the degradation process. Plays a role in a number of cellular processes such as nuclear transport, DNA replication and repair, mitosis and signal transduction. Covalent attachment to its substrates requires prior activation by the E1 complex SAE1-SAE2 and linkage to the E2 enzyme UBE2I, and can be promoted by an E3 ligase such as PIAS1-4, RANBP2 or CBX4.,online information:SUMO protein entry,PTM:Cleavage of precursor form by SENP1 or SENP2 is necessary for function.,PTM:Cleavage of precursor form by SENP1, SENP2 or SENP5 is necessary for function.,PTM:Polymeric chains can be formed through Lys-11 cross-linking.,similarity:Belongs to the ubiquitin family. SUMO subfamily.,similarity:Contains 1 ubiquitin-like domain.,subcellular location:Nuclear bodies.,subunit:Homotrimer (Potential). Crystal packing analysis suggests a possible trimeric assembly, of which the biological significance remains to be determined. Interacts with SAE2 and UBE2I. Covalently attached to a number of proteins. Interacts with PELP1.,subunit:Interacts with SAE2 and UBE2I. Covalently attached to a number of proteins.,tissue specificity:Broadly expressed.,tissue specificity:Expressed predominantly in liver.,
show all
Function:
proteolysis, macromolecule catabolic process, protein sumoylation, modification-dependent protein catabolic process,protein catabolic process, protein modification by small protein conjugation, modification-dependent macromolecule catabolic process, cellular protein catabolic process, cellular macromolecule catabolic process, proteolysis involved in cellular protein catabolic process, protein modification by small protein conjugation or removal,
show all
Cellular Localization:
Nucleus. Nucleus, PML body.
show all
Tissue Expression:
show all
Reference Citation({{totalcount}})
Catalog: KA3976C
Size
Price
Status
Qty.
96well
$330.00
In stock
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}