Catalog: KA3968C
Size
Price
Status
Qty.
96well
$330.00
In stock
0
Add to cart


Collected


Collect
Main Information
Reactivity
Human
Applications
ELISA
Conjugate/Modification
Unmodified
Detailed Information
Storage
2-8°C/6 months,Ship by ice bag
Modification
Unmodified
Detection Method
Colorimetric
Related Products
Antigen&Target Information
Gene Name:
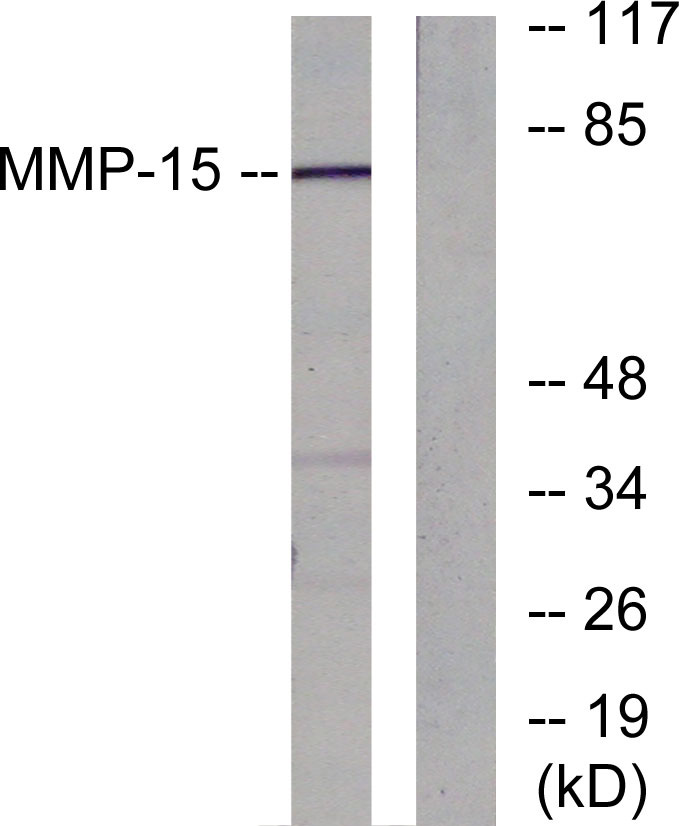
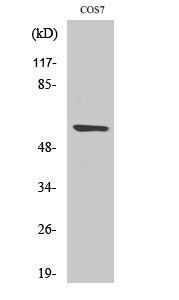
MMP15
show all
Other Name:
Matrix metalloproteinase-15 ;
MMP-15 ;
Membrane-type matrix metalloproteinase 2 ;
MT-MMP 2 ;
MTMMP2 ;
Membrane-type-2 matrix metalloproteinase ;
MT2-MMP ;
MT2MMP ;
SMCP-2 ;
MMP-15 ;
Membrane-type matrix metalloproteinase 2 ;
MT-MMP 2 ;
MTMMP2 ;
Membrane-type-2 matrix metalloproteinase ;
MT2-MMP ;
MT2MMP ;
SMCP-2 ;
show all
Background:
cofactor:Binds 1 zinc ion per subunit.,cofactor:Calcium.,domain:The conserved cysteine present in the cysteine-switch motif binds the catalytic zinc ion, thus inhibiting the enzyme. The dissociation of the cysteine from the zinc ion upon the activation-peptide release activates the enzyme.,function:Endopeptidase that degrades various components of the extracellular matrix. May activate progelatinase A.,PTM:The precursor is cleaved by a furin endopeptidase.,similarity:Belongs to the peptidase M10A family.,similarity:Contains 4 hemopexin-like domains.,tissue specificity:Appeared to be synthesized preferentially in liver, placenta, testis, colon and intestine. Substantial amounts are also detected in pancreas, kidney, lung, heart and skeletal muscle.,
show all
Function:
proteolysis,
show all
Cellular Localization:
Membrane ; Single-pass type I membrane protein ; Extracellular side .
show all
Reference Citation({{totalcount}})
Catalog: KA3968C
Size
Price
Status
Qty.
96well
$330.00
In stock
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}