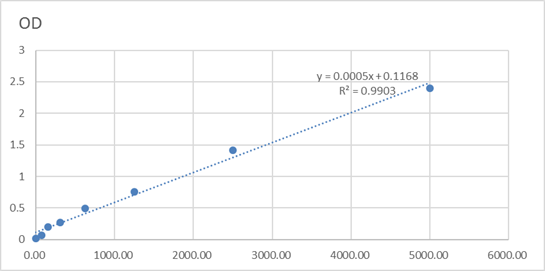
Catalog: KA1319C
Size
Price
Status
Qty.
96well
$470.00
In stock
0
Add to cart


Collected


Collect
Main Information
Reactivity
Human, Mouse, Rat
Applications
ELISA
Conjugate/Modification
Phospho
Detailed Information
Storage
2-8°C/6 months,Ship by ice bag
Modification
Phospho
Detection Method
Colorimetric
Related Products
Antigen&Target Information
Gene Name:
PDE4B/PDE4C/PDE4D
show all
Other Name:
cAMP-specific 3',5'-cyclic phosphodiesterase 4D ;
DPDE3 ;
PDE43 ;
DPDE3 ;
PDE43 ;
show all
Background:
catalytic activity:Adenosine 3',5'-cyclic phosphate + H(2)O = adenosine 5'-phosphate.,cofactor:Binds 2 divalent metal cations per subunit. Site 1 may preferentially bind zinc ions, while site 2 has a preference for magnesium and/or manganese ions.,disease:Genetic variations in PDE4D might be associated with susceptibility to stroke type 1 (STRK1) [MIM:606799]. A stroke is an acute neurologic event leading to death of neural tissue of the brain and resulting in loss of motor, sensory and/or cognitive function. PubMed:17006457 states that association with stroke has to be considered with caution.,enzyme regulation:Inhibited by rolipram. Activated by phosphatidic acid.,function:Regulates the levels of cAMP in the cell.,pathway:Purine metabolism; cAMP degradation; AMP from cAMP: step 1/1.,PTM:Isoform 2 and isoform 11 are activated by phosphorylation (in vitro), but not isoform 8. Isoform 7 and isoform 12 are phosphorylated on Ser-49, Ser-51, Ser-55 and Ser-59.,similarity:Belongs to the cyclic nucleotide phosphodiesterase family.,subcellular location:Found in the soluble fraction, associated with membranes, and associated with the cytoskeleton and the centrosome.,subunit:Homodimer for the long isoforms. Isoforms with truncated N-termini are monomeric. Isoform 2 is part of a ternary complex containing PRKAR2A, PRKAR2B and AKAP9. Interacts with PDE4DIP (By similarity). Isoform 5 binds GNB2L1 via its unique N-terminus. Binds ARRB2.,tissue specificity:Widespread; most abundant in skeletal muscle. Isoform 8 is detected in brain. Isoform 9 is detected in brain, placenta, lung and kidney. Isoform 11 is detected in heart and skeletal muscle.,
show all
Function:
muscle system process, purine nucleotide metabolic process, purine nucleotide catabolic process, cAMP catabolic process, muscle contraction, smooth muscle contraction, nucleoside monophosphate metabolic process, nucleoside monophosphate catabolic process, nucleotide catabolic process, cyclic nucleotide metabolic process, cyclic nucleotide catabolic process, nucleobase, nucleoside, nucleotide and nucleic acid catabolic process, nucleobase, nucleoside and nucleotide catabolic process, nitrogen compound catabolic process, cAMP metabolic process, heterocycle catabolic process,
show all
Cellular Localization:
Apical cell membrane . Cytoplasm . Membrane . Cytoplasm, cytoskeleton . Cytoplasm, cytoskeleton, microtubule organizing center, centrosome . Found in the soluble fraction, associated with membranes, and associated with the cytoskeleton and the centrosome (By similarity). Colocalized with SHANK2 to the apical membrane of colonic crypt cells. .
show all
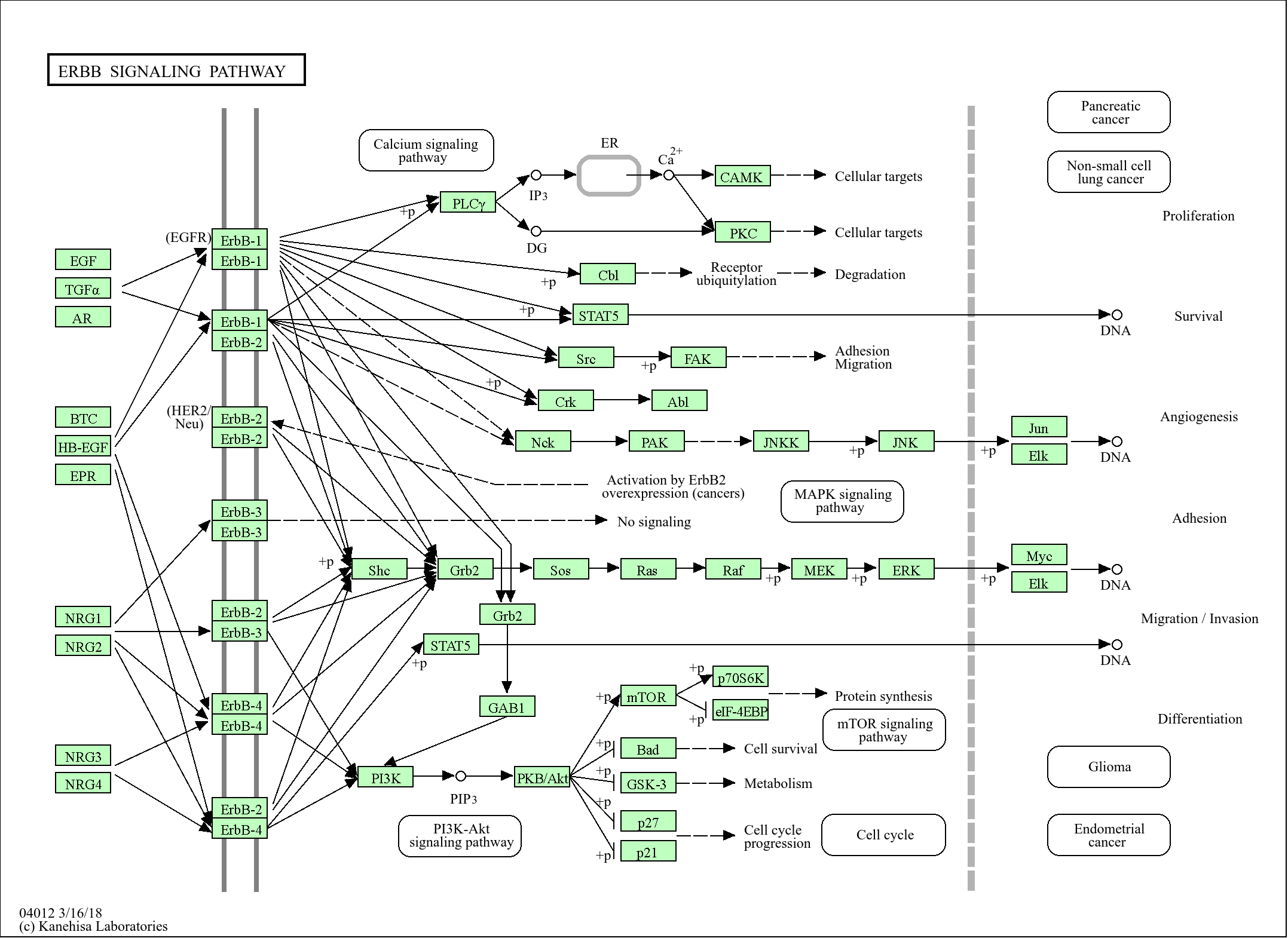
Signaling Pathway
Reference Citation({{totalcount}})
Catalog: KA1319C
Size
Price
Status
Qty.
96well
$470.00
In stock
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}