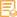Catalog: KA1273C
Size
Price
Status
Qty.
96well
$470.00
In stock
0
Add to cart


Collected


Collect
Main Information
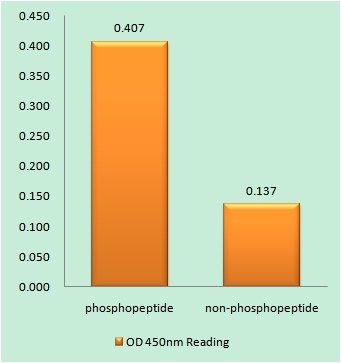
Reactivity
Human, Mouse, Rat
Applications
ELISA
Conjugate/Modification
Phospho
Detailed Information
Storage
2-8°C/6 months,Ship by ice bag
Modification
Phospho
Detection Method
Colorimetric
Related Products
Antigen&Target Information
Gene Name:
CBL
show all
Other Name:
E3 ubiquitin-protein ligase CBL ;
Casitas B-lineage lymphoma proto-oncogene ;
Proto-oncogene c-Cbl ;
RING finger protein 55 ;
Signal transduction protein CBL ;
Casitas B-lineage lymphoma proto-oncogene ;
Proto-oncogene c-Cbl ;
RING finger protein 55 ;
Signal transduction protein CBL ;
show all
Background:
disease:Can be converted to an oncogenic protein by deletions or mutations that disturb its ability to down-regulate RTKs.,domain:The N-terminus is composed of the phosphotyrosine binding (PTB) domain, a short linker region and the RING-type zinc finger. The PTB domain, which is also called TKB (tyrosine kinase binding) domain, is composed of three different subdomains: a four-helix bundle (4H), a calcium-binding EF hand and a divergent SH2 domain.,domain:The RING-type zinc finger domain mediates binding to an E2 ubiquitin-conjugating enzyme.,function:Participates in signal transduction in hematopoietic cells. Adapter protein that functions as a negative regulator of many signaling pathways that start from receptors at the cell surface. Acts as an E3 ubiquitin-protein ligase, which accepts ubiquitin from specific E2 ubiquitin-conjugating enzymes, and then transfers it to substrates promoting their degradation by the proteasome. Recognizes activated receptor tyrosine kinases, including PDGFA, EGF and CSF1, and terminates signaling.,miscellaneous:This protein has one functional calcium-binding site.,pathway:Protein modification; protein ubiquitination.,PTM:Phosphorylated on tyrosine residues by EGFR, SYK, FYN and ZAP70 (By similarity). Phosphorylated on tyrosine residues by INSR.,similarity:Contains 1 CBL N-terminal domain.,similarity:Contains 1 RING-type zinc finger.,similarity:Contains 1 SH2 domain.,similarity:Contains 1 UBA domain.,similarity:Contains 2 EF-hand-like domains.,subunit:Associates with NCK via its SH3 domain. The phosphorylated C-terminus interacts with CD2AP via its second SH3 domain. Binds to UBE2L3. Interacts with adapters SLA, SLA2 and with the phosphorylated C-terminus of SH2B2. Interacts with EGFR, SYK and ZAP70 via the highly conserved Cbl-N region. Also interacts with SORBS1 and INPPL1/SHIP2. Interacts with phosphorylated LAT2. May interact with CBLB.,
show all
Function:
proteolysis, cell surface receptor linked signal transduction, enzyme linked receptor protein signaling pathway,transmembrane receptor protein tyrosine kinase signaling pathway, epidermal growth factor receptor signaling pathway, macromolecule catabolic process, protein ubiquitination, modification-dependent protein catabolic process,regulation of endocytosis, protein catabolic process, protein modification by small protein conjugation, modification-dependent macromolecule catabolic process, cellular protein catabolic process, cellular macromolecule catabolic process, positive regulation of endocytosis, regulation of receptor-mediated endocytosis, positive regulation of receptor-mediated endocytosis, positive regulation of transport, positive regulation of cellular component organization, proteolysis involved in cellular protein catabolic process, regulation of vesicle-mediated transport,protein modification by small protein conjugation or removal,
show all
Cellular Localization:
Cytoplasm. Cell membrane. Cell projection, cilium . Golgi apparatus . Colocalizes with FGFR2 in lipid rafts at the cell membrane.
show all
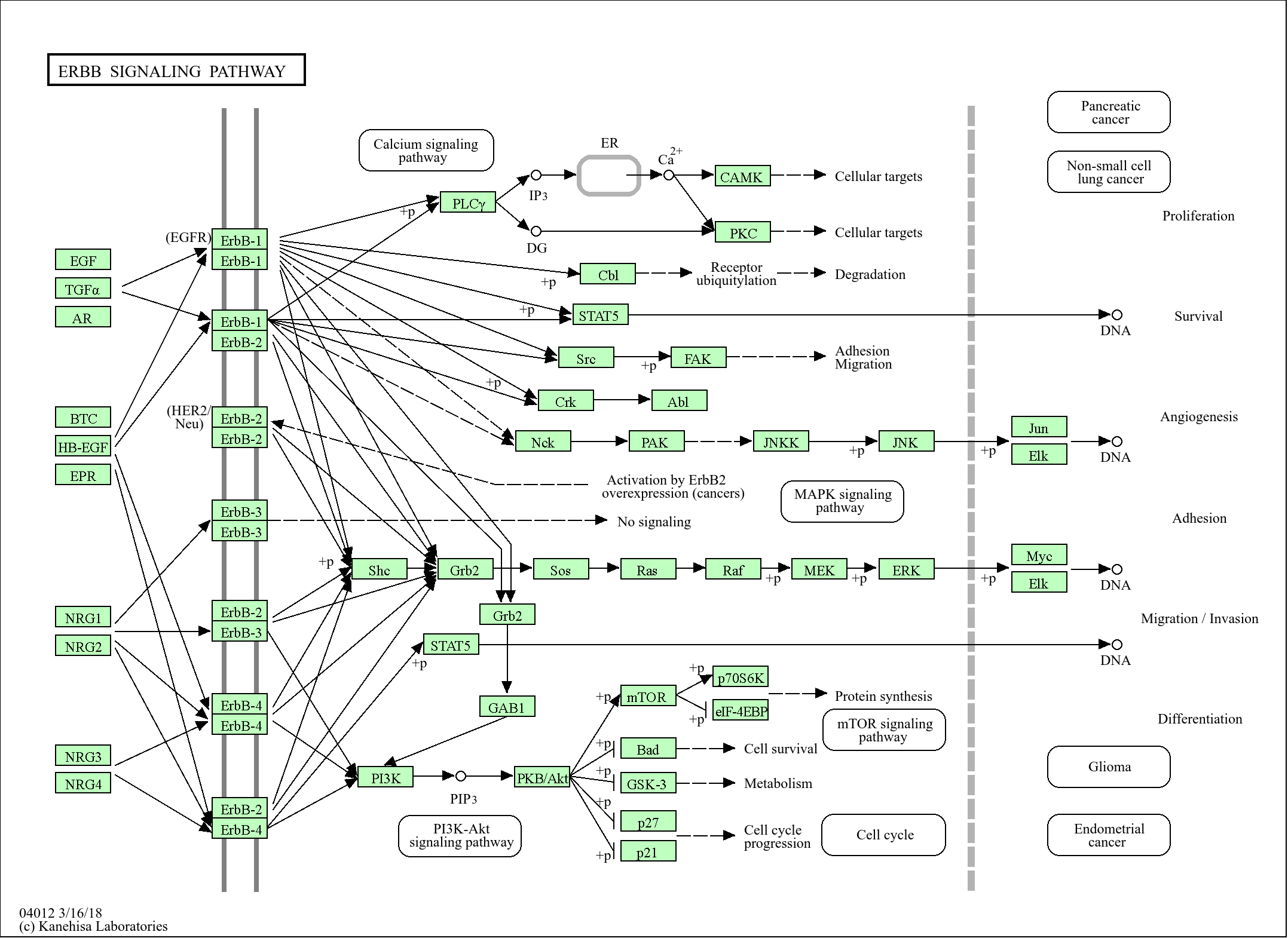
Signaling Pathway
Organismal Systems >> Endocrine system >> Insulin signaling pathway
Human Diseases >> Cancer: overview >> Pathways in cancer
Human Diseases >> Cancer: specific types >> Chronic myeloid leukemia
Environmental Information Processing >> Signal transduction >> ErbB signaling pathway
Genetic Information Processing >> Folding, sorting and degradation >> Ubiquitin mediated proteolysis
Reference Citation({{totalcount}})
Catalog: KA1273C
Size
Price
Status
Qty.
96well
$470.00
In stock
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}