Catalog: KE1242
Size
Price
Status
Qty.
5*96well
$1,850.00
3 weeks
0
2*96well
$740.00
3 weeks
0
96well
$370.00
3 weeks
0
Add to cart


Collected


Collect
Main Information
Reactivity
Human
Applications
ELISA
Conjugate/Modification
Unmodified
Detailed Information
Specificity
Sample Type for Cell Culture Supernates, Cell lysates, Tissue Lysates, Serum, EDTA Plasma, Heparin Plasma
Storage
2-8°C/6 months,Ship by ice bag
Modification
Unmodified
Sample Type
Sample Type for Cell Culture Supernates, Cell lysates, Tissue Lysates, Serum, EDTA Plasma, Heparin Plasma
Detection Method
Colorimetric
Sensitivity
250-16000 pg/ml
Related Products
Antigen&Target Information
Specificity:
Sample Type for Cell Culture Supernates, Cell lysates, Tissue Lysates, Serum, EDTA Plasma, Heparin Plasma
show all
Gene Name:
CTSB CPSB
show all
Protein Name:
Cathepsin B
show all
Other Name:
CTSB ;
CPSB ;
Cathepsin B ;
APP secretase ;
APPS ;
Cathepsin B1 ;
[Cleaved into: Cathepsin B light chain ;
Cathepsin B heavy chain]
CPSB ;
Cathepsin B ;
APP secretase ;
APPS ;
Cathepsin B1 ;
[Cleaved into: Cathepsin B light chain ;
Cathepsin B heavy chain]
show all
Background:
catalytic activity:Hydrolysis of proteins with broad specificity for peptide bonds. Preferentially cleaves -Arg-Arg-|-Xaa bonds in small molecule substrates (thus differing from cathepsin L). In addition to being an endopeptidase, shows peptidyl-dipeptidase activity, liberating C-terminal dipeptides.,function:Thiol protease which is believed to participate in intracellular degradation and turnover of proteins. Has also been implicated in tumor invasion and metastasis.,similarity:Belongs to the peptidase C1 family.,subcellular location:Identified by mass spectrometry in melanosome fractions from stage I to stage IV.,subunit:Dimer of a heavy chain and a light chain cross-linked by a disulfide bond.,
show all
Function:
proteolysis, response to wounding, regulation of cell death, regulation of apoptosis, regulation of programmed cell death,
show all
Cellular Localization:
Lysosome . Melanosome . Secreted, extracellular space . Apical cell membrane ; Peripheral membrane protein ; Extracellular side . Identified by mass spectrometry in melanosome fractions from stage I to stage IV (PubMed:17081065). Localizes to the lumen of thyroid follicles and to the apical membrane of thyroid epithelial cells (By similarity). .
show all
Tissue Expression:
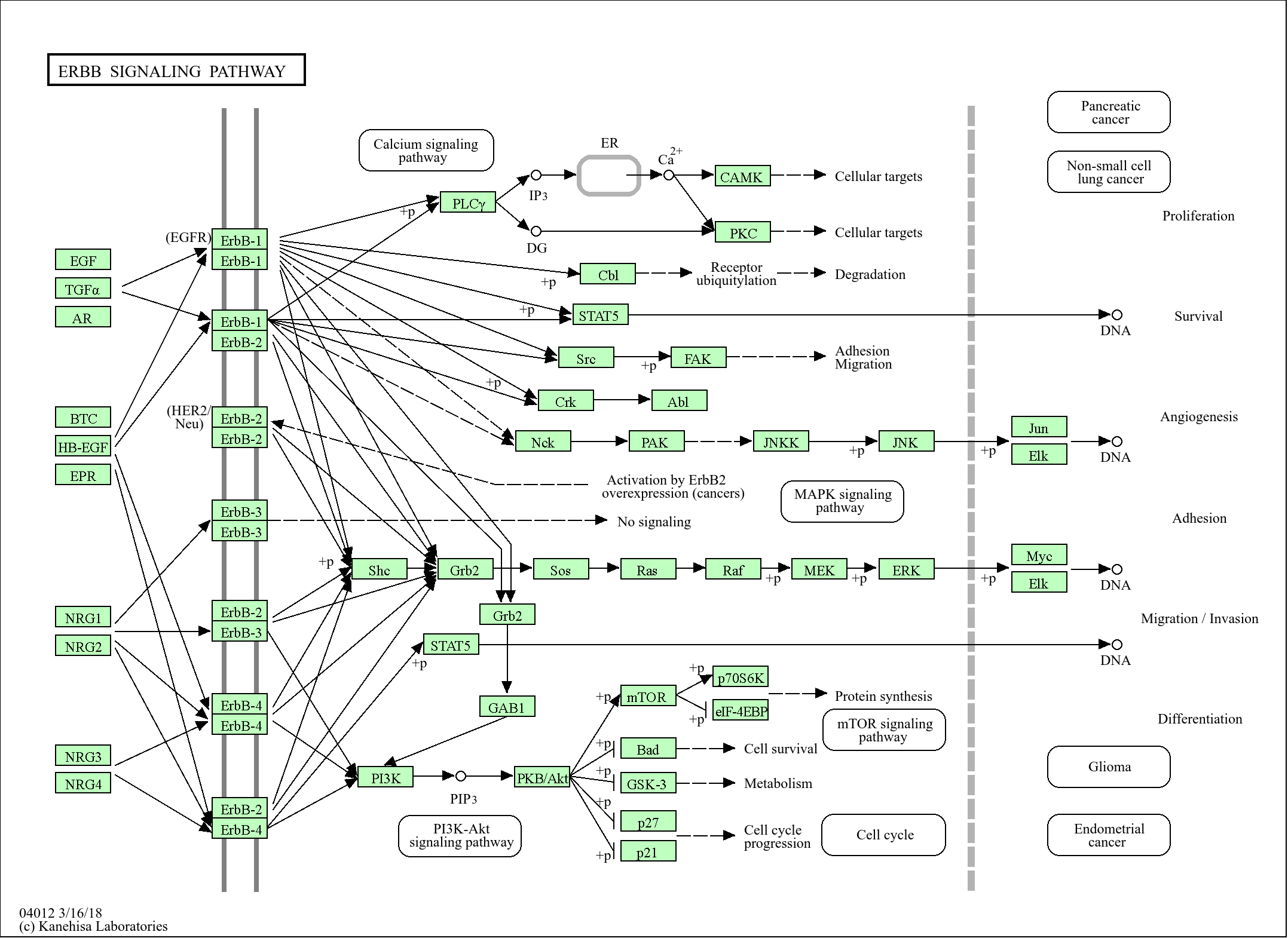
Signaling Pathway
Cellular Processes >> Transport and catabolism >> Lysosome
Cellular Processes >> Transport and catabolism >> Autophagy - animal
Cellular Processes >> Cell growth and death >> Apoptosis
Organismal Systems >> Immune system >> NOD-like receptor signaling pathway
Organismal Systems >> Immune system >> Antigen processing and presentation
Reference Citation({{totalcount}})
Catalog: KE1242
Size
Price
Status
Qty.
5*96well
$1,850.00
3 weeks
0
2*96well
$740.00
3 weeks
0
96well
$370.00
3 weeks
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}