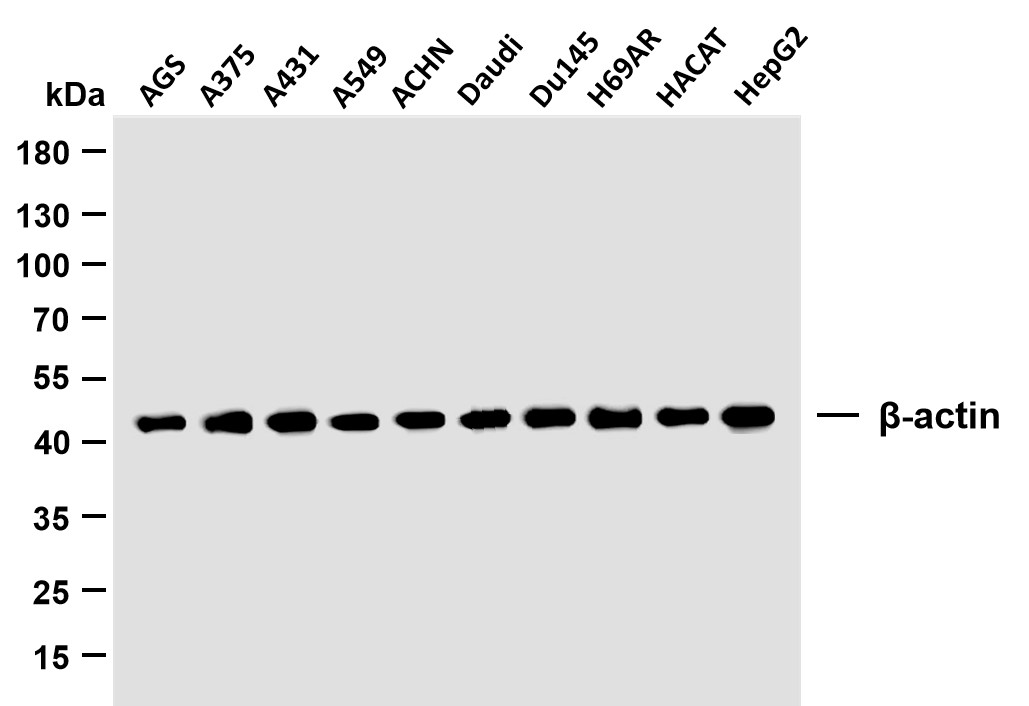
Catalog: YM8749
Size
Price
Status
Qty.
200μL
$600.00
3 weeks
0
100μL
$340.00
3 weeks
0
40μL
$190.00
3 weeks
0
Add to cart


Collected


Collect
Main Information
Target
ACC
Host Species
Rabbit
Reactivity
Human, Mouse, Rat
Applications
WB, IHC, IF, IP, ELISA
MW
266kD (Calculated)
266kD (Observed)
Conjugate/Modification
Phospho
Detailed Information
Recommended Dilution Ratio
IHC 1:200-1:1000; WB 1:1000-1:15000; IF 1:200-1:1000; ELISA 1:5000-1:20000; IP 1:50-1:200;
Formulation
PBS, 50% glycerol, 0.05% Proclin 300, 0.05%BSA
Specificity
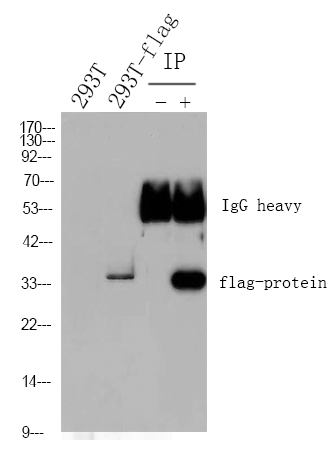
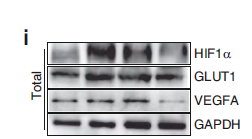
Acetyl-CoA Carboxylase (Phospho Ser79) Monoclonal Antibody detects endogenous levels of ACC protein only when phosphorylated at S79.The name of modified sites may be influenced by many factors, such as species (the modified site was not originally found in human samples) and the change of protein sequence (the previous protein sequence is incomplete, and the protein sequence may be prolonged with the development of protein sequencing technology). When naming, we will use the "numbers" in historical reference to keep the sites consistent with the reports. The antibody binds to the following modification sequence (lowercase letters are modification sites):SSmSG
Purification
Protein A
Storage
-15°C to -25°C/1 year(Do not lower than -25°C)
MW(Calculated)
266kD
MW(Observed)
266kD
Modification
Phospho
Clonality
Monoclonal
Clone Number
PT0983R
Isotype
IgG, Kappa
Related Products
Antigen&Target Information
Specificity:
Acetyl-CoA Carboxylase (Phospho Ser79) Monoclonal Antibody detects endogenous levels of ACC protein only when phosphorylated at S79.The name of modified sites may be influenced by many factors, such as species (the modified site was not originally found in human samples) and the change of protein sequence (the previous protein sequence is incomplete, and the protein sequence may be prolonged with the development of protein sequencing technology). When naming, we will use the "numbers" in historical reference to keep the sites consistent with the reports. The antibody binds to the following modification sequence (lowercase letters are modification sites):SSmSG
show all
Gene Name:
ACACB;ACACA
show all
Protein Name:
ACC,Acetyl-CoA carboxylase 2;ACC-beta;Acetyl-CoA carboxylase 1;ACC1;
show all
Other Name:
ACACA ;
ACAC ;
ACC1 ;
ACCA ;
Acetyl-CoA carboxylase 1 ;
ACC1 ;
ACC-alpha ;
ACACB,Acetyl CoA carboxylase 2,ACC beta,ACC2,ACCB,AcetylCoA carboxylase 2,ACCbeta,ACCβ,ACC β,
ACAC ;
ACC1 ;
ACCA ;
Acetyl-CoA carboxylase 1 ;
ACC1 ;
ACC-alpha ;
ACACB,Acetyl CoA carboxylase 2,ACC beta,ACC2,ACCB,AcetylCoA carboxylase 2,ACCbeta,ACCβ,ACC β,
show all
Database Link:
Background:
Acetyl-CoA carboxylase (ACC) is a complex multifunctional enzyme system. ACC is a biotin-containing enzyme which catalyzes the carboxylation of acetyl-CoA to malonyl-CoA, the rate-limiting step in fatty acid synthesis. ACC-beta is thought to control fatty acid oxidation by means of the ability of malonyl-CoA to inhibit carnitine-palmitoyl-CoA transferase I, the rate-limiting step in fatty acid uptake and oxidation by mitochondria. ACC-beta may be involved in the regulation of fatty acid oxidation, rather than fatty acid biosynthesis. There is evidence for the presence of two ACC-beta isoforms. [provided by RefSeq, Jul 2008],
show all
Function:
Catalytic activity:ATP + acetyl-CoA + HCO(3)(-) = ADP + phosphate + malonyl-CoA.,Catalytic activity:ATP + biotin-carboxyl-carrier protein + CO(2) = ADP + phosphate + carboxybiotin-carboxyl-carrier protein.,cofactor:Binds 2 manganese ions per subunit.,cofactor:Biotin.,enzyme regulation:Activated by citrate. Inhibited by malonyl-CoA.,Function:ACC-beta may be involved in the provision of malonyl-CoA or in the regulation of fatty acid oxidation, rather than fatty acid biosynthesis. Carries out three functions: biotin carboxyl carrier protein, biotin carboxylase and carboxyltransferase.,pathway:Lipid metabolism; malonyl-CoA biosynthesis; malonyl-CoA from acetyl-CoA: step 1/1.,similarity:Contains 1 ATP-grasp domain.,similarity:Contains 1 biotin carboxylation domain.,similarity:Contains 1 biotinyl-binding domain.,similarity:Contains 1 carboxyltransferase domain.,subcellular location:May associate with membranes.,tissue specificity:Predominantly expressed in the heart, skeletal muscles and liver.,
show all
Cellular Localization:
Mitochondrion
show all
Tissue Expression:
Widely expressed with highest levels in heart, skeletal muscle, liver, adipose tissue, mammary gland, adrenal gland and colon (PubMed:9099716). Isoform 3 is expressed in skeletal muscle, adipose tissue and liver (at protein level) (PubMed:19190759). Isoform 3 is detected at high levels in adipose tissue with lower levels in heart, liver, skeletal muscle and testis (PubMed:19190759).
show all
Research Areas:
>>Fatty acid biosynthesis ;
>>Pyruvate metabolism ;
>>Propanoate metabolism ;
>>Metabolic pathways ;
>>AMPK signaling pathway ;
>>Insulin signaling pathway ;
>>Adipocytokine signaling pathway ;
>>Glucagon signaling pathway ;
>>Insulin resistance ;
>>Alcoholic liver disease
>>Pyruvate metabolism ;
>>Propanoate metabolism ;
>>Metabolic pathways ;
>>AMPK signaling pathway ;
>>Insulin signaling pathway ;
>>Adipocytokine signaling pathway ;
>>Glucagon signaling pathway ;
>>Insulin resistance ;
>>Alcoholic liver disease
show all
Reference Citation({{totalcount}})
Catalog: YM8749
Size
Price
Status
Qty.
200μL
$600.00
3 weeks
0
100μL
$340.00
3 weeks
0
40μL
$190.00
3 weeks
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}